
A new operative technique for dissecting perforator vessel in perforator flap: a better way to minimize donor-site morbidity
Introduction
Perforator flap consists of skin and/or subcutaneous fat. The vessels that supply blood to the flap are isolated perforator vessels. Those perforators may pass either through or in between the deep tissues (mostly muscle) (1). The perforator flap has enabled to avoid sacrifice of the muscle, the main vessels and deep fascia and to minimize donor site morbidity by meticulous dissection and anastomosis (2). Perforator flaps have been performed in increasing numbers since Koshima and Soeda first described perforator flaps in 1989 (3). The main advantage of this technique is that it achieves better accuracy in reconstruction and at the same time minimizes donor-site morbidity. Numbers of clinical series have been reported that perforator flap can be used to realize the really defect replace “like with like” (4-6).
However, the application of perforator flap requires a skillful microsurgical technique and a steep long learning curve, which limits the application of perforator flap (7-9). The elevation of the flap requires meticulous attention to detail and a high degree of flexibility of the operative plan according to the position, size, and presence of vascular pedicle (10,11). The disadvantages of perforator flaps depend on their technical demands and lead to potential increase in operative time. In additional, dissection of the perforator vessel may be difficult because of anatomical variability of vascular pedicle (6,12).
The dissection of perforator vessels is the key role of success elevation flap (13). Anterograde approaches and retrograde approaches are the main two operative procedures for the perforator dissection (14-16). However, Anterograde dissection needs to explore the main trunk vascular, and then along those vessels to dissect the perforator, this procedure is blindness and often results in a perforator and nerve injure (15,17). Although retrograde dissection has been exhibited in most of literature, both blunt dissection and cutting off the muscle were adopted in the procedure of the conventional retrograde dissection. This procedure often results in higher donor site morbidity, such as a sensory deficit, muscle weakness and limitation of limb activities (13,16).
To overcome the limitation of traditional retrograde dissection of perforator vessel, we have modified the technical tips basing on our 15 years of experience on application of perforator flap. The purpose of this study was to introduce our experience on a new operative technique for dissecting perforator vascular in the perforator flap and explore its clinical outcome.
Methods
From June 2013 to November 2016, 119 patients with soft tissue defects underwent reconstruction with the anterolateral thigh perforator flaps (ALTPs). All patients in this series had exposure of underlying vital structures including vessels, nerves, bones, and tendons. The procedures were performed by a senior author (J Tang) at Hand & Microsurgery department of Xiangya Hospital of South Central University. Clinical data were collected based on our institutional database. The study followed the ethical guidelines of the Hospital Ethical Committee of the Xiangya Hospital. The individuals in this manuscript have given written informed consent to publish these case details. Demographics were recorded, including patient age, sex and length of follow up. Data were also collected on flap-related characterize, flap success rate, re-exposure rate, postoperative flap-related complications and donor-site morbidity.
Preoperative planning and flap design
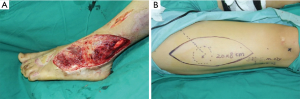
It is essential procedure for perforator flap surgery with an accurate preoperative planning (10,14). Preoperative planning begins with evaluation of the soft defects to provide a specific customized reconstruction (Figure 1). On the one hand, the surgeon should consider both the dimensions and constituent component of the defect; on the other hand, the most appropriate donor site, the location of perforator vessels and the length of vascular pedicle need to be meticulous consideration. We usually used the tomography-assisted angiography (CTA) scan to assess the vascular anatomy of the recipient site (18,19). A hand holder Doppler was used to locate the perforator in the donor site. After debridement, a paper template with the same dimensions as defect was created. According to the shape of the wound, the laxity of the skin over the anterolateral aspect of the thigh and the location of perforator vessels, the perforator flap was outline on the donor site.
Operative technique
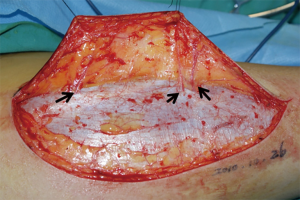
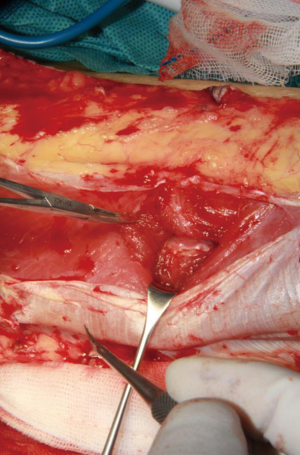
The surgical technique was showed through elevating an ALTP flap in the paper. Patients were placed in the supine position with the leg straight in a neutral position. Based on the perforators mapped and three-dimensional features of the wound, the flap was outlined on the anterolateral aspect of the thigh. Initially, only one paddle side is incised to avoid the flap elevation failure, because the position of perforator maybe varied, and it is enable to permit alteration of the skin paddle according to the location of vessels selected. The flap was harvest at the suprafascial plane. The surgeon should preserve several additional perforator vessels until the main perforator was identified (Figure 2). And then, opening the deep fascia was performed to explore the deep perforator vessel course. It is necessary to incise the deep fascia with enough wide in order to explore the vessels in the surgical, because an adequate exposure can reduce the risks damaging of the perforator vessel.
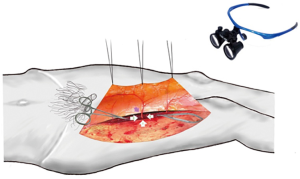
Dissecting the intramuscular course of perforator vessel is the most difficult procedure. The surgeon should use loupe magnification to assist in dissection the perforator vessels at this step (Figure 3). In a first phase, the side of the perforator vessel, which is adjacent the surgeon, was dissected to expose the course of the pedicle through the muscle (Figure 4). This step is preferentially performed in the same direction as the underlying muscle fibers and toward. The muscle fibers were split by sharp dissection. According to the loose connective tissue cuff around the perforator vessels, the path was indicated to through the muscle and where the muscle fibers need to be split. We did not cease to dissect and trace the perforator vessel until identified the source vessels.
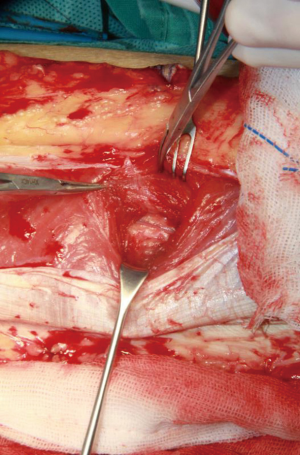
The next step, the left and right side of the perforator vessel was dissected. 3mm width facial tissue surrounding the perforator should be retained to avoid perforator injury (Figure 5). And then, the medial edge of the flap was incised, and the flap dissection proceeded in a medial to lateral direction at the superficial fascia plane. The incision was ceased until encounter the perforator vessel. The fourth sides of the perforator vessel then was dissected and traced to the main trunk vascular (Figure 6). Every side branch should be taken careful to coagulated, ligated, or clipped to prohibit a hemostasis. Notable, at least 1 to 2 mm away from the perforator should be preserved to avoid to injure the perforator vessel and allow hemostasis to be rescued if there is continued bleeding.
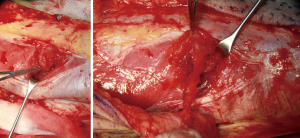
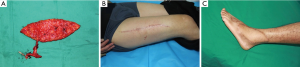
The perforator was traced back to the main trunk of the descending branch of the lateral circumflex femoral artery (LCFA), which was dissected according to pedicle length requirements. Once the dominant perforator vessel supplying the flap was completely dissected, a vascular clamp was used to identify blood supply of the flap. After the flap was transferred to the defect, the wound of the donor site was closed directly after complete hemostasis and reliable drainage (Figure 7).
Results
The ALTP flaps were successfully elevated to reconstruct soft tissue defect for 119 patients, including 98 males and 21 females. The average of the years was 40.58. The size of skin paddle was 120.59±59.31 cm2. A total of 148 perforators were included in the ALTP flaps (84 percent perforators were musculocutaneous perforator, 16 percent perforators were septocutaneous perforator), the average of number of perforators were 1.24. The mean flap elevation time was 60.85±20.25 minutes. The success rate of flap elevation was 98%, only three perforators were injured and one perforator occurred vasospasm during the operation. The addition of papaverine was used to alleviate the problem. Five cases, including three venous crisis and two artery crisis, presented with vascular crisis after flap transfer. The rate of vascular crisis was 4.2%. The vascular crisis was alleviated by re-exploration. All of flaps were completely survived except one flap had partial flap necrosis. The mean follow-up time was 13.32±8.9 months, most of cases showed satisfactory contour, and there was no excessive bulk. No muscular weakness was displayed in this group.
Discussion
Complex soft tissue defect reconstruction always is a challenge problem for the plastic and reconstruction surgeon (4,10). Conventional flap transfer often displayed bulking contour, unsatisfactory color match, unstable mobile surface, poor function recovery and higher donor site morbidity (10). In the past decades, more and more perforator flaps have been performed for defect reconstruction due to achieve the defect replace “like with like” and minimize the donor site morbidity (1,20). Perforator flap have become the workhorse procedure in the soft tissue reconstruction (21,22). However, ALTP flap is known for variations of its vascular pedicle. Failure to understand its variability can easier lead to vascular flap embarrassment and tissue loss (23). Several authors described that 5 to 6 percent of patients absent suitable cutaneous perforator for free ALTP transfer in the thigh (24,25). Many surgeons have found that it is very difficult to elevate a perforator flap because of variations in the size and location of the cutaneous perforators (5,26). Meanwhile, it is known that the type of perforator vessel usually belongs to musculocutaneous and not septocutaneous perforators (27,28). Consequently, elevation flap frequently requires meticulous intramuscular dissection. In our series cases, the results displayed that 84 percent perforators were musculocutaneous perforator. Therefore, application of the perforator flap requires long learning curve and skillful microsurgery technique. In the present study, we introduced a new dissection technique for harvesting flap by orderly retrograde four side dissection.
We found that this technique was easier and safer than the conventional technique. In our present study, 148 perforators were included in the paper, only three perforators were injured and one occurred vasospasm. Donor-site morbidity of the perforator flap can be produced with multiple factors, including damage to the muscle fiber or nerve, closure with skin grafts and injure to the deep fascia. Comparing with the conventional dissection technique, orderly retrograde four side dissection technique has presented number of advantages. On the one hand, fewer muscle fibers are sacrificed and the dissection is smoother with this operative technique; on the other hand, this procedure facilitates the pedicle manipulation and improves the visualization of all side branches and motor nerves (Figure 8). It reduces the risk factor for perforator avulsion during manipulation. Furthermore, It is known that the motor nerves often be encountered at the level of the deeper vessels, often just underneath or within the deeper part of the muscle. This dissection technique can explore clearly visualization to avoid damage the nerve.
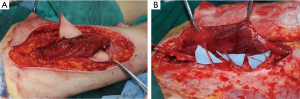
Although many advantages of the dissection technique have been demonstrated in this paper, some technical tips and disadvantages also should be taken care. Firstly, Perforator selection is based on the type of perforator vessels, location within the flap, vessel diameter, and flap zones to be used. Intramuscular septa perforator vessel always is preferentially choose for the perforator flap. Secondly, the deep fascia should be incises enough wide to explore the deeper vessel and avoid to damage the perforator. Thirdly, the perforator flaps was spared with deeper structures to reduce the donor site morbidity. The small perforators are more likely to produce vasospasm and twisting. Therefore, the dissection procedure should be flexible as much as possible. In additional, the intramuscular dissection should be performed in the same direction as the underlying muscle fibers and toward. The loose connective tissue cuff around the perforator vessels can indicate the path to through the muscle.
Conclusions
Orderly retrograde four sides dissection of the perforator vessels in the perforator flap can provide with less donor site morbidity, shorter operative time and safer than the traditional methods. It is a reliable operation for elevation the perforator flap.
Acknowledgments
Funding: This publication was funded in part by the National Natural Science Foundation of China (81472104).
Footnote
Provenance and Peer Review: The article was commissioned by the editorial office, Journal of Xiangya Medicine for the series “Perforator Flap”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2018.09.06). The series “Perforator Flap” was commissioned by the editorial office without any funding or sponsorship. JYT served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Hospital Ethical Committee of the Xiangya Hospital (No. 201403117) and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Blondeel PN, Van Landuyt KH, Monstrey SJ, et al. The "Gent" consensus on perforator flap terminology: preliminary definitions. Plast Reconstr Surg 2003;112:1378-83; quiz 1383, 1516; discussion 1384-7.
- Geddes CR, Morris SF, Neligan PC. Perforator flaps: evolution, classification, and applications. Ann Plast Surg 2003;50:90-9. [Crossref] [PubMed]
- Koshima I, Soeda S. Inferior epigastric artery skin flaps without rectus abdominis muscle. Br J Plast Surg 1989;42:645-8. [Crossref] [PubMed]
- Zhang YX, Hayakawa TJ, Levin LS, et al. The Economy in Autologous Tissue Transfer: Part 1. The Kiss Flap Technique. Plast Reconstr Surg 2016;137:1018-30. [Crossref] [PubMed]
- Saint-Cyr M, Wong C, Schaverien MV, et al. The Perforasome Theory: Vascular Anatomy and Clinical Implications. Plast Reconstr Surg 2009;124:1529-44. [Crossref] [PubMed]
- Saint-Cyr M, Schaverien M, Wong C, et al. The extended anterolateral thigh flap: anatomical basis and clinical experience. Plast Reconstr Surg 2009;123:1245-55. [Crossref] [PubMed]
- Lakhiani C, DeFazio MV, Han K, et al. Donor-Site Morbidity Following Free Tissue Harvest from the Thigh: A Systematic Review and Pooled Analysis of Complications. J Reconstr Microsurg 2016;32:342-57. [Crossref] [PubMed]
- Knott PD, Seth R, Waters HH, et al. Short-term donor site morbidity: A comparison of the anterolateral thigh and radial forearm fasciocutaneous free flaps. Head Neck 2016;38:E945-8. [Crossref] [PubMed]
- Chen YC, Scaglioni MF, Carrillo Jimenez LE, et al. Suprafascial Anterolateral Thigh Flap Harvest: A Better Way to Minimize Donor-Site Morbidity in Head and Neck Reconstruction. Plast Reconstr Surg 2016;138:689-98. [Crossref] [PubMed]
- Kim JT, Kim SW. Perforator Flap versus Conventional Flap. J Korean Med Sci 2015;30:514-22. [Crossref] [PubMed]
- Mukherjee MK, Parwaz M, Chakravarty B, et al. Perforator flap: A novel method for providing skin cover to lower limb defects. Med J Armed Forces India 2012;68:328-34. [Crossref] [PubMed]
- Zachara M, Drozdowski P, Wysocki M, et al. Anatomical variability of the anterolateral thigh flap perforators between sexes: a cadaveric study. Eur J Plast Surg 2013;36:179-84. [Crossref] [PubMed]
- Dancey A, Blondeel PN. Technical tips for safe perforator vessel dissection applicable to all perforator flaps. Clin Plast Surg 2010;37:593-606. xi-vi. [Crossref] [PubMed]
- Lee YC, Chiu HY, Shieh SJ. The clinical application of anterolateral thigh flap. Plast Surg Int 2011;2011:127353. [Crossref] [PubMed]
- Adler N, Dorafshar AH, Agarwal JP, et al. Harvesting the lateral femoral circumflex chimera free flap: guidelines for elevation. Plast Reconstr Surg 2009;123:918-25. [Crossref] [PubMed]
- Gravvanis A, Niranjan NS. Retrograde dissection of the vascular pedicle of deep inferior epigastric artery perforator (DIEAP) flap. Ann Plast Surg 2008;60:395-7. [Crossref] [PubMed]
- Zhao J, Chan FC, Yang X, et al. Salvage Free Anterolateral Thigh Composite Flap Transfer Based on the Musculocutaneous Perforator Retrograde Blood Flow Principle. J Craniofac Surg 2016;27:e178-81. [Crossref] [PubMed]
- Qing L, Wu P, Liang J, et al. Use of Flow-Through Anterolateral Thigh Perforator Flaps in Reconstruction of Complex Extremity Defects. J Reconstr Microsurg 2015;31:571-8. [Crossref] [PubMed]
- Tang J, Fang T, Song D, et al. Free deep inferior epigastric artery perforator flap for reconstruction of soft-tissue defects in extremities of children. Microsurgery 2013;33:612-9. [Crossref] [PubMed]
- Lin CT, Wang CH, Ou KW, et al. Clinical applications of the pedicled anterolateral thigh flap in reconstruction. ANZ J Surg 2017;87:499-504. [Crossref] [PubMed]
- Seidenstuecker K, van Waes C, Munder BI, et al. DIEAP flap for safe definitive autologous breast reconstruction. Breast 2016;26:59-66. [Crossref] [PubMed]
- Blondeel PN, Morris SF, Hallock GG, et al. Perforator Flaps: Anatomy, Technique and Clinical Applications. St Louis (MO): QMP, 2006.
- Yang X, Zhang G, Liu Y, et al. Vascular anatomy and clinical application of anterolateral leg perforator flaps. Plast Reconstr Surg 2013;131:534e-43e. [Crossref] [PubMed]
- Kuo YR, Seng-Feng J, Kuo FM, et al. Versatility of the free anterolateral thigh flap for reconstruction of soft-tissue defects: review of 140 cases. Ann Plast Surg 2002;48:161-6. [Crossref] [PubMed]
- Koshima I, Fukuda H, Utunomiya R, et al. The anterolateral thigh flap; variations in its vascular pedicle. Br J Plast Surg 1989;42:260-2. [Crossref] [PubMed]
- Saint-Cyr M, Schaverien M, Arbique G, et al. Three- and four-dimensional computed tomographic angiography and venography for the investigation of the vascular anatomy and perfusion of perforator flaps. Plast Reconstr Surg 2008;121:772-80. [Crossref] [PubMed]
- Taylor GI, Rozen WM, Whitaker IS. Establishing a perforator flap nomenclature based on anatomical principles. Plast Reconstr Surg 2012;129:877e-9e. [Crossref] [PubMed]
- Taylor GI, Corlett RJ, Dhar SC, et al. The Anatomical (Angiosome) and Clinical Territories of Cutaneous Perforating Arteries: What Goes around Comes Around. Plast Reconstr Surg 2011;127:1447-59. [Crossref] [PubMed]
Cite this article as: Qing L, Wu P, Bing Z, Yu F, Pang X, Ding P, Bing X, Lei Z, Fu J, Tang J. A new operative technique for dissecting perforator vessel in perforator flap: a better way to minimize donor-site morbidity. J Xiangya Med 2018;3:39.


