
Comparison of survival to hospital discharge predictions in elderly traumatic brain injury using CHID, CIT, and CART algorithms
Highlight box
Key findings
• The tree-based algorithms performed well in terms of prognosis in older traumatic brain injury (TBI) patients, with high sensitivity.
What is known and what is new?
• Elderly people who have suffered a TBI are at risk of an unfavorable outcome and a poor prognosis.
• The paper evaluates the prognostic power of several tree-based algorithms for older patients’ survival to hospital discharge after TBI.
What is the implication, and what should change now?
• The tree-based models are simple and user-friendly, making them appropriate for use as screening tools in general practice.
• These tools may support physicians to establish personalized treatment strategies and advise patients’ families about their prognosis.
Introduction
Traumatic brain injury (TBI) in the elderly has become a major problem in various countries because the trend of population aging will accelerate in the upcoming decades (1). The incidence of TBI among the elderly is rising progressively. Tayler et al. compared the total hospitalizations of TBI patients aged 65–74 years old between 2007 and 2013 in the United States and found that the hospitalization rate increased from 126.5 to 139.4 per 100,000 population. The TBI-related hospitalization in patients aged ≥75 years old increased from 356.9 per 100,000 population in 2007 to 454.4 per 100,000 population in 2013. In addition, mortality of TBI patients aged 65–74 and ≥75 years old ranged between 24.3–24.4 per 100,000 population and 66.3–76.1 per 100,000 population (2). Bobeff et al. evaluated 214 elderly TBI patients and found that survival to hospital release was 59.8%, whereas 14-day and 30-day mortality rates were 11.2% and 19.2%, respectively (3).
Age has been recognized as one of the prognostic factors from the literature review (4,5). According to prior systematic review and meta-analysis, the geriatric population exhibited an overall mortality rate of 38.3% and mortality is highly associated with the advanced age of the patients (6). Therefore, prognostication in elderly TBI could assist physicians in developing individualized rehabilitation and treatment plans that enhance quality of life, improve prognosis, and minimize medical expenditures, particularly in limited-resource settings.
Various clinical prediction tools (CPTs) have been performed for prognostication in TBI based on a review of the literature, but there is a lack of evidence explored in geriatric TBI. Wang used various machine learning (ML) algorithms to predict the prognosis of elderly TBI and found that area under the receiver operating characteristic (ROC) curve (AUC) ranged from 0.792 to 0.799 (7). However, the advanced ML-based tools may be limited for usage in general practice because the CPTs require skills and expertise.
The tree-based techniques such as Chi-square Automation Interaction Detection (CHAID), Conditional Inference Trees (CIT), and Classification and Regression Tree (CART) are some of the common algorithms that render predictive models highly accurate and user-friendly in various fields of medicine. CHAID is a classification algorithm that uses Chi-square statistics to discover optimal splits in decision trees. Turcato et al. (8) utilized CHAID to predict the risk of cerebral bleeding in patients taking oral anticoagulants after mild TBI and AUC was 0.802, while Allen et al. used this algorithm for predicting recovery in patients with sport-related concussion that had AUC was 0.80 (9).
CIT is a non-parametric decision tree that selects the predictors by permutation-based significance tests (10). In Thailand, Nalita et al. proposed the CIT for predicting the prognosis of pediatric medulloblastoma (11). Moreover, the CART algorithm has been used to predict the clinical outcomes of both classification (categorical outcome) and regression (numeric outcome) problems from the literature review. Tunthanathip et al. classified high-risk patients who are at increased likelihood of developing surgical site infections after neurosurgical procedures using CART with an AUC of 0.64–0.71 (12). Furthermore, Wang et al. predicted 30-day mortality in elderly TBI using a decision tree algorithm with an AUC of 0.712 (7).
From the literature review, few studies use tree-based algorithms to predict prognosis in TBI, particularly in the elderly. Moreover, the fact that tree-based models are simple to use and understand has challenged us to use various tree-based models for predicting the prognosis of elderly TBI. The objective study was to compare the predictability among CHAID, CIT, and CART algorithms for survival to hospital discharge prediction in elderly TBI. We present this article in accordance with the TRIPOD reporting checklist (available at https://jxym.amegroups.com/article/view/10.21037/jxym-23-39/rc).
Methods
Study designs and study population
The present study was carried out as a retrospective cohort study and TBI patients aged 60 years and above were admitted to a tertiary hospital between January 2015 and January 2023. In total, 3,929 patients were consecutively included in the study, and 513 patients who did not have a cranial computed tomography (CT) scan or patients who died before arrival were excluded from the study. Therefore, 3,416 TBI patients were reviewed their medical records, and the patient’s clinical characteristics, imaging results, and survival status before hospital discharge were collected.
The AUC formula was used to calculate sample size and various parameters based on Wang et al. (7) were calculated as follows: AUC of 0.799, alpha of 0.05, and estimation error of 0.05 (13). Therefore, the sample size of the study population was at least 370 patients.
Definition
Various definitions of old age were observed from the literature review (14,15). According to the World Health Organization (WHO) definition, the elderly were defined as individuals aged 60 years and older, and the age categories of TBI patients were as follows: 60–64, 65–69, 70–74, 75–79, and ≥80 years of age (14).
For analysis, demographic data and cranial CT scan results were extracted from the patient’s medical record. Systemic hypotension in the present study was defined as systolic blood pressure less than 90 mmHg or diastolic blood pressure less than 60 mmHg (16). In addition, because hypotension causes a misunderstanding of the Glasgow Coma Scale (GCS) score due to insufficient cerebral perfusion, the GCS score collected in the current study was the patient’s GCS score after emergency department resuscitation. Therefore, the severity of TBI was categorized based on GCS score as mild (GCS scores 13–15), moderate (GCS scores 9–12), and severe (GCS scores 3–8) (17). The cranial CT results, the type of cerebral hemorrhage, the skull fracture, the midline displacement, and the obliteration of the basal cistern were evaluated by two neurosurgeons. Furthermore, survival to hospital discharge was defined as the condition in which a patient is alive before the date of their discharge from the hospital, regardless of their functional outcome.
Ethical considerations
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The human research ethics committee of Faculty of Medicine, Prince of Songkla University approved the present study (REC 65-138-10-1). The informed consent of the patients was not necessary for the present study because it was a retrospective analysis. However, patient identification numbers were encoded before analysis.
Statistical analysis
The workflow diagram of the present investigation is illustrated in Figure 1. Descriptive analysis was used to determine the baseline clinical characteristics, mechanism of injury, and intracranial injury after cranial CT scans of the entire dataset. Continuous variables were represented with mean and standard deviation (SD), whereas categorical variables were represented with frequencies and percentages.

By utilizing a random data splitting technique, 70% of the entire dataset was allocated for training the predictive models, while the remaining 30% was reserved for evaluating the models’ performance (18). Before the analysis, the complete case approach was utilized to manage missing values.
For the feature selection and model development, CHAID, CIT, and CART algorithms were performed to select the predictors from various clinical characteristics and imaging findings from the training dataset. The CHAID technique uses a Chi-square measurement metric to identify the most significant feature and construct a tree-based classification model (19), while CART’s tree model in the present study was built using the Gini index for binary splitting with 5-fold cross-validation (10,12,18). In addition, the CIT algorithm assesses the true effect of predictors that had maximum Gini index by utilizing permutation-based significance tests (10).
Therefore, the tree-based models for the prediction of survival to hospital discharge from CHAID, CIT, and CART algorithms were validated by the testing dataset.
Survival to hospital discharge was the endpoint of the current investigation, which was binary classifiers; thus, the cutoff point of 0.5 or greater of predictive probability was employed to forecast a predicted survival. Moreover, the predictive performance of each algorithm was evaluated utilizing the following metrics: sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), accuracy, and F1 score. In addition, the ROC curves with AUC were also determined (20). Based on prior studies, an AUC of 0.7 indicated acceptable performance, 0.8 represented excellent performance, and 0.9 indicated great performance (21,22). The R version 4.4.0 software was applied for the analysis (R Foundation, Vienna, Austria).
Results
A total of 3,416 patients were included, with 51.1% of them being male. The average age of the present cohort was 74.75 years (SD 9.22), and 32.3% of all TBI patients were 80 years of age or older. There was a history of aspirin use in 9.2%, clopidogrel use in 2.0%, and warfarin use in 1.5% of the entire data. Based on the mechanism of injury, ground-level falls accounted for 65.6% of TBI cases, traffic injuries for 27.3%, and falls from heights for 3.1% of cases. When their distribution according to severity was examined, 92.8% had mild TBI, 3.6% had moderate TBI, and 3.6% had severe TBI. Subdural hematoma (SDH) was the most prevalent intracranial injury, accounting for 14.0% of all cases, while diffuse axonal injury was found in 1.1%. As a result, the in-hospital mortality of the total cohort was 1.1%. In addition, Table 1 demonstrates baseline clinical characteristics according to the training and testing datasets.
Table 1
| Characteristics | Training dataset (n=2,391) | Testing dataset (n=1,025) | Total dataset (n=3,416) |
|---|---|---|---|
| Age group (years) | 74.73±9.14 | 74.78±9.43 | 74.75±9.22 |
| 60–64 | 362 (15.1) | 180 (17.6) | 542 (15.9) |
| 65–69 | 445 (18.6) | 174 (17.0) | 619 (18.1) |
| 70–74 | 407 (17.0) | 169 (16.5) | 576 (16.9) |
| 75–79 | 412 (17.2) | 164 (16.0) | 576 (16.9) |
| ≥80 | 765 (32.0) | 338 (33.0) | 1,103 (32.3) |
| Gender (male) | 1,192 (49.9) | 552 (53.9) | 1,744 (51.1) |
| Mechanism of injury | |||
| Ground-level fall | 1,583 (66.2) | 685 (66.8) | 2,241 (65.6) |
| Fall from height | 70 (2.9) | 36 (3.5) | 106 (3.1) |
| Motorcycle crash | 539 (22.5) | 254 (24.8) | 793 (23.2) |
| Car crash | 56 (2.3) | 32 (3.1) | 88 (2.6) |
| Pedestrian injury | 38 (1.6) | 13 (1.3) | 51 (1.5) |
| Object stuck at head | 48 (2.0) | 16 (1.6) | 64 (1.9) |
| Penetrating injury | 5 (0.2) | 1 (0.1) | 6 (0.2) |
| Body assault | 17 (0.7) | 6 (0.6) | 23 (0.7) |
| Bicycle-related injury | 21 (0.9) | 7 (0.7) | 28 (0.8) |
| Blast injury | 2 (0.1) | 0 (0.0) | 2 (0.1) |
| Other | 12 (0.5) | 2 (0.2) | 14 (0.4) |
| Traffic injury | 633 (26.5) | 299 (29.2) | 932 (27.3) |
| Medication | |||
| Aspirin | 212 (8.9) | 103 (10.0) | 315 (9.2) |
| Clopidogrel | 47 (2.0) | 20 (2.0) | 67 (2.0) |
| Warfarin | 41 (1.7) | 11 (1.1) | 52 (1.5) |
| Signs and symptoms | |||
| Loss of consciousness | 129 (5.4) | 63 (6.1) | 192 (5.6) |
| Amnesia | 133 (5.6) | 64 (6.2) | 197 (5.8) |
| Seizure | 22 (0.9) | 16 (1.6) | 38 (1.1) |
| Motor weakness | 8 (0.3) | 5 (0.5) | 13 (0.4) |
| Hypotension | 14 (0.6) | 8 (0.8) | 22 (0.6) |
| Glasgow Coma Scale score | |||
| 13–15 | 2,223 (93.0) | 947 (92.4) | 3,170 (92.8) |
| 9–12 | 90 (3.8) | 34 (3.3) | 124 (3.6) |
| 3–8 | 78 (3.3) | 44 (4.3) | 122 (3.6) |
| Pupillary light reflex | |||
| Fixed both eyes | 18 (0.8) | 8 (0.8) | 26 (0.8) |
| Fixed one eye | 20 (0.8) | 19 (1.9) | 39 (1.1) |
| React both eyes | 2,353 (98.4) | 998 (97.4) | 3,351 (98.1) |
| Cranial computed tomography finding | |||
| Skull fracture | 47 (2.0) | 30 (2.9) | 77 (2.3) |
| Basilar skull fracture | 56 (2.3) | 35 (3.4) | 91 (2.7) |
| Intracerebral hematoma | |||
| Epidural hematoma | 47 (2.0) | 30 (2.9) | 77 (2.3) |
| Subdural hematoma | 304 (12.7) | 174 (17.0) | 478 (14.0) |
| Contusion | 207 (8.7) | 99 (9.7) | 306 (9.0) |
| Subarachnoid hemorrhage | 260 (10.9) | 126 (12.3) | 386 (11.3) |
| Intraventricular hemorrhage | 54 (2.3) | 26 (2.5) | 80 (2.3) |
| Brainstem contusion | 3 (0.1) | 2 (0.2) | 5 (0.1) |
| Diffuse axonal injury | 27 (1.1) | 12 (1.2) | 39 (1.1) |
| Basal cistern obliteration | 98 (4.1) | 52 (5.1) | 150 (4.4) |
| Midline shift (mm) | 0.49±2.43 | 0.59±2.58 | 0.52±2.47 |
| <0.5 | 2,234 (93.4) | 946 (92.3) | 3,180 (93.1) |
| ≥0.5 | 157 (6.6) | 79 (7.7) | 236 (6.9) |
| Surgery | |||
| No | 2,264 (94.7) | 958 (93.5) | 3,222 (94.3) |
| Yes | 127 (5.3) | 67 (6.5) | 194 (5.7) |
| Survival to hospital discharge | 2,318 (96.9) | 979 (95.5) | 3,297 (96.5) |
Data are expressed as mean ± standard deviation or n (%).
The training dataset had an average age of 74.73 years (SD 9.14), whereas the testing dataset had an average age of 74.78 years (SD 9.43). In both datasets, the most common mechanism of TBI was a fall to the ground, while traffic injuries were detected in 26.5% and 29.2% of the training and testing datasets, respectively. The GCS score ranging from 3 to 8 was present in 3.3% of the training dataset, while severe TBI of the testing dataset was found in 4.3% of cases. As a result, survival to hospital discharge of the training and testing dataset was 96.9% and 95.5%, respectively.
Model development
A total of 25 variables, including both clinical factors and imaging findings, were utilized to construct the predictive models by CHAID, CIT, and CART algorithms.
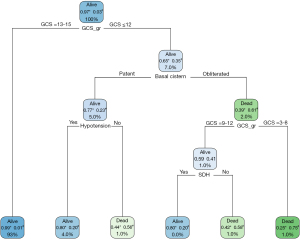
The CHAID model included various predictors such as severity of TBI, midline shift, basilar skull fracture, basal cistern obliteration, surgery, aspirin usage, intraventricular hemorrhage, mechanism of injury, and epidural hematoma, as shown in Figure 2. Figure 3 shows the CIT model included the following: severity of TBI, midline shift, basal cistern obliteration, surgery, mechanism of injury, and epidural hematoma. Additionally, the classification model of CART is demonstrated in Figure 4 which comprises the severity of TBI, basal cistern obliteration, hypotension, and SDH.


Predictability of survival to hospital discharge
A comparison of survival to hospital discharge predictions in among CHAID, CIT, and CART models is shown in Table 2. The tree-based models had high sensitivity to predict survival in elderly TBI. The CART model had the highest sensitivity of 0.99 (95% CI: 0.98–1.00), while CHAID, CIT, and CART models had a specificity of 0.64 (95% CI: 0.45–0.82), 0.73 (95% CI: 0.56–0.90), and 0.47 (95% CI: 0.33–0.62), respectively.
Table 2
| Algorithms | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | Accuracy (95% CI) | F1 score (95% CI) |
|---|---|---|---|---|---|---|
| CHAID | 0.97 (0.95–0.98) | 0.64 (0.45–0.82) | 0.99 (0.98–1.00) | 0.34 (0.21–0.48) | 0.96 (0.95–0.97) | 0.98 (0.96–0.99) |
| CIT | 0.97 (0.96–0.98) | 0.73 (0.56–0.90) | 0.99 (0.98–1.00) | 0.41 (0.27–0.55) | 0.96 (0.95–0.97) | 0.98 (0.97–0.99) |
| CART | 0.99 (0.98–1.00) | 0.47 (0.33–0.62) | 0.97 (0.96–0.98) | 0.75 (0.60–0.91) | 0.96 (0.95–0.98) | 0.98 (0.97–0.99) |
CHAID, Chi-square Automation Interaction Detection; CIT, Conditional Inference Trees; CART, Classification and Regression Trees; TBI, traumatic brain injury; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value.
As shown in Figure 5, the CART model had the highest predictive performance with an AUC of 0.736 (95% CI: 0.641–0.830), whereas the AUCs of CHAID and CIT models were 0.669 (95% CI: 0.573–0.766) and 0.703 (95% CI: 0.607–0.799), respectively.

Discussion
The present study found that the mortality rate for older TBI patients was 1.1%, whereas prior studies found that the mortality rate ranged between 11.2–38.3% (3,6). This could be explained by the fact that the current study’s definition of elderly had a lower age threshold compared to previous studies. According to the WHO’s definition, the process of aging is accelerated by a gradual accumulation of various kinds of molecular and cellular damage in this age threshold. Elderly patients who suffered a TBI were more likely to develop systemic complications, had fewer physical reserves, and had a poor prognosis (23).
Among the CHAID, CIT, and CART models, the prognostic factors associated with survival to hospital discharge comprised severity, mechanism of injury, type of intracranial hematoma, midline shift, and obliteration of basal cistern. These findings are in concordance with other research reports. According to Vathanalaoha et al., low GCS scores have been associated with poor outcomes. Patients with TBI who had a GCS score of 3–5 had a 6-month favorable outcome of 28.2% and a mortality rate of 58.3% (24). The degree of midline shift and basal cistern obliteration have been reported as prognostic factors in TBI in the literature review (25,26). Karnjanasavitree et al. (25) found that obliterated basal cistern was significantly associated with poor functional outcome, while Brazinova et al. observed that closed basal cisterns and midline shifts more than 15 mm were prognostic markers in elderly severe TBI patients (26).
As a result of predictive performance, the CART and CIT algorithms had acceptable performance to predict survival to hospital discharge. According to a previous study, the CART model had an AUC of 0.712 and a sensitivity of 0.425 to predict 30-day mortality in elderly TBI, but the predictive model constructed using the Adoboost algorithm had an AUC of 0.799 and a sensitivity of 0.701 (7). The current study’s CART model was nearly as predictable as previous research, but it also showed a high degree of sensitivity, suggesting that it might be applied as a screening tool in the real world (27). In addition, the predictive model of the tree-based algorithm is presented as a diagram that is easy to apply for general physicians and other health personnel to apply the model in general practice (28).
To the best of the authors’ knowledge, this is the first study that demonstrated and compared the predictive capabilities among tree-based algorithms in the prognosis of elderly TBI. Nevertheless, some limitations should be recognized. An imbalance of endpoints was also identified in this present study; thus, the F1 score was calculated as a method of evaluating predictability. Consequently, all tree-based models achieved F1 scores exceeding 0.9, indicating favorable performance (29). A limited number of fatalities were identified in the present cohort; this may be rectifiable through a multicenter study, which would improve predictive performance. Also, external validation with unseen data in the future is necessary to validate the predictive capabilities of the tree-based models (17,30). In addition, the ability of numerous CPTs to predict clinical outcomes has been studied, including nomograms, scoring systems, and various ML algorithms (30-32). Future research should focus on the comparison of prognostication in predictive models across different CPTs. Finally, elderly individuals with TBI are more likely to have a worse prognosis, a longer hospital stay, and a higcher rehabilitation cost, particular elderly with senile asthenia syndrome or frailty (33-36). The present study did not explore number of this group. In the future, the survival rate of fragile elderly individuals and prognostic factors associated with outcome should be identified to develop the predictive model (37).
Conclusions
In summary, tree-based algorithms demonstrated acceptable prognostication performance in elderly TBI with outstanding sensitivity. These algorithms’ models are straightforward and user-friendly, making them appropriate for use as screening tools in general practice. Future research could be focused on validating these models with unseen data and comparing predictability across different CPTs.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jxym.amegroups.com/article/view/10.21037/jxym-23-39/rc
Data Sharing Statement: Available at https://jxym.amegroups.com/article/view/10.21037/jxym-23-39/dss
Peer Review File: Available at https://jxym.amegroups.com/article/view/10.21037/jxym-23-39/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jxym.amegroups.com/article/view/10.21037/jxym-23-39/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The human research ethics committee of Faculty of Medicine, Prince of Songkla University approved the present study (REC 65-138-10-1). The informed consent of the patients was not necessary for the present study because it was a retrospective analysis. However, patient identification numbers were encoded before analysis.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Centers for Disease Control and Prevention. Older Adults. [Internet]. 2023. [cited 2023 Sep 2]. Available online: https://www.cdc.gov/stillgoingstrong/olderadults/index.html
- Taylor CA, Bell JM, Breiding MJ, et al. Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths - United States, 2007 and 2013. MMWR Surveill Summ 2017;66:1-16. [Crossref] [PubMed]
- Bobeff EJ, Fortuniak J, Bryszewski B, et al. Mortality After Traumatic Brain Injury in Elderly Patients: A New Scoring System. World Neurosurg 2019;128:e129-47. [Crossref] [PubMed]
- Tran A, Saigle V, Manhas N, et al. Association of age with death and withdrawal of life-sustaining therapy after severe traumatic brain injury. Can J Surg 2023;66:E348-55. [Crossref] [PubMed]
- Mosenthal AC, Livingston DH, Lavery RF, et al. The effect of age on functional outcome in mild traumatic brain injury: 6-month report of a prospective multicenter trial. J Trauma 2004;56:1042-8. [Crossref] [PubMed]
- McIntyre A, Mehta S, Aubut J, et al. Mortality among older adults after a traumatic brain injury: a meta-analysis. Brain Inj 2013;27:31-40. [Crossref] [PubMed]
- Wang R, Zeng X, Long Y, et al. Prediction of Mortality in Geriatric Traumatic Brain Injury Patients Using Machine Learning Algorithms. Brain Sci 2023;13:94. [Crossref] [PubMed]
- Turcato G, Zaboli A, Pfeifer N, et al. Decision tree analysis to predict the risk of intracranial haemorrhage after mild traumatic brain injury in patients taking DOACs. Am J Emerg Med 2021;50:388-93. [Crossref] [PubMed]
- Allen JH, Tang AR, Hajdu KS, et al. Predicting early versus late recovery from sport-related concussion using decision tree analysis. J Neurosurg Pediatr 2023;32:9-18. [Crossref] [PubMed]
- Singh A. Conditional Inference Trees. [Internet]. 2018. [cited 2023 Sep 2]. Available online: https://rpubs.com/awanindra01/ctree
- Nalita N, Ratanalert S, Kanjanapradit K, et al. Survival and Prognostic Factors in Pediatric Patients with Medulloblastoma in Southern Thailand. J Pediatr Neurosci 2018;13:150-7. [Crossref] [PubMed]
- Tunthanathip T, Sae-Heng S, Oearsakul T, et al. Machine learning applications for the prediction of surgical site infection in neurological operations. Neurosurg Focus 2019;47:E7. [Crossref] [PubMed]
- Thai Thanh Truc. Statistics and Sample Size Pro. [Internet]. 2020. [cited 2023 Sep 2]. Available online: https://play.google.com/store/apps/details?id=thaithanhtruc.info.sass&hl=en_US
- World Health Organization. Active Ageing: A Policy Framework. [Internet]. 2014. [cited 2023 Sep 12]. Available online: https://extranet.who.int/agefriendlyworld/active-ageing-a-policy-framework/
- Rudnicka E, Napierała P, Podfigurna A, et al. The World Health Organization (WHO) approach to healthy ageing. Maturitas 2020;139:6-11. [Crossref] [PubMed]
- Saengrung S, Kaewborisutsakul A, Tunthanathip T, et al. Risk Factors for Intraoperative Hypotension During Decompressive Craniectomy in traumatic Brain Injury Patients. World Neurosurg 2022;162:e652-8. [Crossref] [PubMed]
- Taweesomboonyat T, Kaewborisutsakul A, Tunthanathip T, et al. Necessity of in-hospital neurological observation for mild traumatic brain injury patients with negative computed tomography brain scans. J Health Sci Med Res 2000;38:267-74.
- Tunthanathip T, Oearsakul T. Comparison of predicted survival curves and personalized prognosis among cox regression and machine learning approaches in glioblastoma. J Med Artif Intell 2023;6:10. [Crossref]
- Dung NC. When You Need Explanation: Decision Tree Algorithms (CART and CHAID). [Internet]. 2023. [cited 2023 Sep 12]. Available online: https://rpubs.com/chidungkt/451329
- Tunthanathip T, Duangsuwan J, Wattanakitrungroj N, et al. Clinical Nomogram Predicting Intracranial Injury in Pediatric Traumatic Brain Injury. J Pediatr Neurosci 2020;15:409-15. [Crossref] [PubMed]
- Roelen CA, Bültmann U, van Rhenen W, et al. External validation of two prediction models identifying employees at risk of high sickness absence: cohort study with 1-year follow-up. BMC Public Health 2013;13:105. [Crossref] [PubMed]
- Tunthanathip T, Sae-Heng S, Oearsakul T, et al. Economic impact of a machine learning-based strategy for preparation of blood products in brain tumor surgery. PLoS One 2022;17:e0270916. [Crossref] [PubMed]
- Jiang L, Zheng Z, Zhang M. The incidence of geriatric trauma is increasing and comparison of different scoring tools for the prediction of in-hospital mortality in geriatric trauma patients. World J Emerg Surg 2020;15:59. [Crossref] [PubMed]
- Vathanalaoha K, Oearsakul T, Tunthanathip T. Predictive Factors of Survival and 6-Month Favorable Outcome of Very Severe Head Trauma Patients; a Historical Cohort Study. Emerg (Tehran) 2017;5:e24. [PubMed]
- Karnjanasavitree W, Phuenpathom N, Tunthanathip T. The Optimal Operative Timing of Traumatic Intracranial Acute Subdural Hematoma Correlated with Outcome. Asian J Neurosurg 2018;13:1158-64. [Crossref] [PubMed]
- Brazinova A, Mauritz W, Leitgeb J, et al. Outcomes of patients with severe traumatic brain injury who have Glasgow Coma Scale scores of 3 or 4 and are over 65 years old. J Neurotrauma 2010;27:1549-55. [Crossref] [PubMed]
- Maxim LD, Niebo R, Utell MJ. Screening tests: a review with examples. Inhal Toxicol 2014;26:811-28. [Crossref] [PubMed]
- Eschweiler GW, Czornik M, Herrmann ML, et al. Presurgical Screening Improves Risk Prediction for Delirium in Elective Surgery of Older Patients: The PAWEL RISK Study. Front Aging Neurosci 2021;13:679933. [Crossref] [PubMed]
- Allwright S. What is a good F1 score and how do I interpret it? [internet]. 2023 [cited 2011 Jul 5]. Available online: https://stephenallwright.com/good-f1-score/
- Kaewborisutsakul A, Tunthanathip T. Development and internal validation of a nomogram for predicting outcomes in children with traumatic subdural hematoma. Acute Crit Care 2022;37:429-37. [Crossref] [PubMed]
- Trakulpanitkit A, Tunthanathip T. Comparison of intracranial pressure prediction in hydrocephalus patients among linear, non-linear, and machine learning regression models in Thailand. Acute Crit Care 2023;38:362-70. [Crossref] [PubMed]
- Tunthanathip T, Duangsuwan J, Wattanakitrungroj N, et al. Comparison of intracranial injury predictability between machine learning algorithms and the nomogram in pediatric traumatic brain injury. Neurosurg Focus 2021;51:E7. [Crossref] [PubMed]
- Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146-56. [Crossref] [PubMed]
- Lin HS, Watts JN, Peel NM, et al. Frailty and post-operative outcomes in older surgical patients: a systematic review. BMC Geriatr 2016;16:157. [Crossref] [PubMed]
- Savva GM, Donoghue OA, Horgan F, et al. Using timed up-and-go to identify frail members of the older population. J Gerontol A Biol Sci Med Sci 2013;68:441-6. [Crossref] [PubMed]
- Hoogendijk EO, van der Horst HE, Deeg DJ, et al. The identification of frail older adults in primary care: comparing the accuracy of five simple instruments. Age Ageing 2013;42:262-5. [Crossref] [PubMed]
- Tunthanathip T, Phuenpathom N, Jongjit A. Web-based calculator using machine learning to predict intracranial hematoma in geriatric traumatic brain injury. J Hosp Manag Health Policy 2023;7:16. [Crossref]
Cite this article as: Tunthanathip T, Phuenpathom N, Jongjit A. Comparison of survival to hospital discharge predictions in elderly traumatic brain injury using CHID, CIT, and CART algorithms. J Xiangya Med 2024;9:6.


