
The Holter characteristics of vagal-mediated paroxysmal atrial fibrillation and clinical observation of low-level vagus nerve stimulation after radiofrequency ablation
Highlight box
Key findings
• During the ablation process of isolating the pulmonary veins, high-frequency fragmented potentials were observed, followed by a transition to sinus rhythm; however, at this point, the pulmonary veins were not yet isolated. Patients who experienced this phenomenon and received additional low-level vagus nerve stimulation (LIVNS) postoperatively had good clinical outcomes.
What is known and what is new?
• LIVNS can treat atrial fibrillation (AF) by regulating vagal nerve tone, but it may not be effective for all patients with AF.
• Theoretically, patients with vagally mediated AF may have better outcomes after radiofrequency ablation when LIVNS is used postoperatively. This study defines vagally mediated AF based on responses during radiofrequency ablation and designs a clinical controlled trial to verify through follow-up that patients in this category can achieve better prognoses with the addition of LIVNS after the procedure.
What is the implication, and what should change now?
• By selecting suitable cases, LIVNS can have a greater effect; however, this requires more clinical data support and advancements in the technique of LIVNS itself.
Introduction
Previous literature has shown that some patients experience significant vagal reactions during the catheter ablation procedure for atrial fibrillation (AF). These reactions manifest as lengthening of the RR interval, nausea and vomiting. The ablation catheter recorded the transmission of accelerated high-frequency fragmented signals at the site of ablation. The necrosis of the corresponding ablated ganglia resulted in the heart rhythm becoming more regular or restoring sinus rhythm. The results of the current study indicate the trigger to be the participation of the vagal ganglia in the pathogenesis of AF. Vagal nerve response plays a crucial role in the pathogenesis of AF. Identifying and treating patients with vagal-mediated AF is a clinically significant and unresolved issue.
Methods
Clinical data
This study included 126 patients with paroxysmal AF (PAF) who underwent catheter ablation between June 2020 and June 2023. The inclusion criteria were: (I) availability of Holter examination results obtained during the week prior to surgery, demonstrating more than 2,000 episodes of premature atrial contractions (PACs) within 24 h; (II) indications for catheter ablation of AF without contraindications, resulting in a successful procedure that allowed for postoperative follow-up. Exclusion criteria included severe renal insufficiency, age over 80 years or under 30 years, presence of cardiac pacemakers, concomitant myocardial disease, and patients who were deceased or lost to follow up.
The collection and categorization of research indicators
Vagal-mediated PAF was defined as follows: during the surgical procedure, AF may persist, and during the isolation of the pulmonary veins, high-frequency fractional potentials are observed, transitioning to sinus rhythm even before complete pulmonary vein isolation (Figure 1). Among our cohort, vagal-mediated PAF (the vagal type) was observed in 43 cases, accounting for 34.1% of the total, while non-vagal-mediated PAF (the common type) was seen in 83 cases, accounting for 65.9%.

For Holter recording, two criteria were established: (I) more than 2,000 PACs must occur within 24 h; (II) the duration of AF or atrial flutter recorded within 24 h must be less than 10 minutes. Data were collected to analyze the hourly occurrence of atrial premature beats, the total number of PACs over 24 h, and the proportion of PACs (defined as hourly PAC count divided by the total PACs over 24 h).
Radiofrequency ablation and follow-up
The radiofrequency ablation procedure was conducted as follows: all patients received non-vitamin K antagonist oral anticoagulants (NOACs). Transesophageal echocardiography was performed to rule out thrombus formation prior to ablation. Radiofrequency catheter ablation (RFCA) was conducted under the guidance of the ENSITE system (Abbott, Chicago, IL, USA). All patients underwent bilateral circumferential pulmonary vein electrical isolation, with bidirectional block of the pulmonary veins serving as the procedural endpoint. Autonomic potentials were fully ablated following pulmonary vein electrical isolation.
The follow-up protocol included re-evaluating Holter recordings 72 h post-operation and every 3 months thereafter. In cases of clinical symptoms, electrocardiograms (ECGs) were re-examined immediately. Defined outcomes included AF recurrence during follow-up, characterized as AF lasting more than 30 seconds, as captured in ECG or Holter monitoring, occurring after a 3-month blanking period.
For the low-level vagus nerve stimulation (LIVNS) intervention, vagus nerve stimulation was administered using a Huatuo vagus nerve stimulator (model TENS-200, Suzhou Medical Supplies Co., Ltd., Suzhou, China). The output current was set to 1 mA, with a pulse frequency of 20 Hz and pulse width ≤1 ms for treatment. Patients were prescribed oral amiodarone for 3 months post-operation, followed by sustained-release metoprolol tablets for an additional 9 months, with NOACs continued for at least 3 months. Patients were randomly assigned to either the drug + LIVNS group or the drug-only group. LIVNS stimulation was initiated three days post-operation via an ear vagus nerve stimulator, administered three times daily for 15 minutes until the end of the follow-up period. An informative study flow chart is presented in Figure 2.

Statistical analysis
Data processing was performed using the R4.0 statistical programming language. Categorical variables were expressed as frequencies, while continuous data were presented as means with standard deviations. Statistical analyses included the Chi-squared test for categorical data and t-tests for continuous data. Survival analysis was conducted using the Kaplan-Meier method.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Dongguan Kanghua Hospital, Dongguan City, Guangdong Province, China (No. 2021024) and informed consent was obtained from all individual participants.
Results
Baseline data
There were statistically insignificant differences between the two groups in terms of age, sex, history of hypertension/AF, left atrial diameter measured under the long-axis view of echocardiography, as well as the total number of PACs per the Hotler recordings (Table 1).
Table 1
| Variables | Vagus type | Common type | P value |
|---|---|---|---|
| Group, n (%) | 43 (34.1) | 83 (65.9) | – |
| Age (years), mean ± SD | 66.47±9.59 | 66.70±8.87 | 0.89 |
| Sex, n (%) | 0.88 | ||
| Male | 23 (53.5) | 42 (50.6) | |
| Female | 20 (46.5) | 41 (49.4) | |
| Duration of HTN (months), mean ± SD | 13.44±8.58 | 13.08±9.81 | 0.83 |
| Duration of PAF (months), mean ± SD | 21.70±16.58 | 26.75±18.99 | 0.89 |
| LA diameter (mm), mean ± SD | 38.20±4.17 | 38.54±4.53 | 0.68 |
| PACs, mean ± SD | 8,274.74±2,719.86 | 7,772.78±2,712.27 | 0.32 |
SD, standard deviation; HTN, hypertension; PAF, paroxysmal atrial fibrillation; LA, left atrial; PACs, premature atrial contractions.
Distribution of PACs
In a 24-h period, certain regularities were observed in the distribution of PACs in the vagal type. The number of PACs during the time intervals of 0–1, 1–2, 12–13, 13–14, 19–20, and 20–21 were higher compared with the other time intervals. However, this regularity was not observed in the common type (Figure 3). The results of the t-test analysis on the number of PACs during these six time intervals showed a significant statistical difference between the vagal type and common type (P<0.01).

Similarly, the graphical analysis of the percentage of time-interval PACs (number of PACs in a time interval/the total number of PACs in 24 h) revealed similar observations. There was a statistically significant difference between the vagal type and the common type regarding the percentage of PACs in the time intervals of 0–1, 1–2, 12–13, 13–14, 19–20, and 20–21 (Figure 4). These six specific time intervals were referred to as the vagus hyperactivity period.

Comparison of the percentage of PACs during the vagus hyperactivity period between vagal type and common type
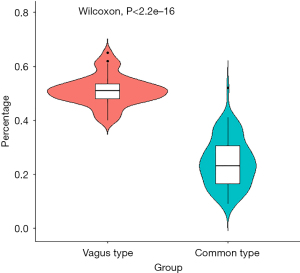
The PACs observed in the vagal hyperactivity period were divided by the PACs in a 24-h period to determine the percentage of PACs during the vagal hyperactivity period, followed by a t-test analysis to statistically analyze the differences in the percentage of PACs during the vagal hyperactivity period among the two groups. The results showed that the vagal type was associated with a statistically significant higher percentage of PACs during the vagal hyperactivity period compared with the common type (0.51±0.06 vs. 0.24±0.09) (Figure 5).
Distribution of autonomic potentials in pulmonary veins of vagal and common type patients after pulmonary vein electrical isolation
The autonomic potentials after pulmonary vein electrical isolation were recorded in 21 cases of the vagal type (49%) and 25 cases of the common type (30%). In the vagal type, 81% of the autonomic potentials were recorded in the bilateral superior pulmonary veins compared with 56% in the common type (Figure 6).

Analysis of Holter recordings 72 h after operation
All patients underwent the analysis of the Holter recordings 72 h after operation. The results showed that PACs were recorded at >2,000 beats/24 h in 8 patients with the vagal type, and PAF was recorded in 5 patients with the vagal type. The average duration was 2.6±1.5 h, while no PACs with >2,000 beats/24 h or PAF were recorded in the common type.
Effect of LIVNS on postoperative follow-up
A total of 18 patients (42%) in the vagus type and 33 patients (40%) in the common type were assigned to the drug combined with the LIVNS group. During the follow-up of 666.72±275.40 days, PAF was found to have recurred in 8 patients in the vagus type, all in the drug subgroup. In the common type, 21 patients had AF recurrence (follow-up period of 630.61±245.63 days), of which 6 were in the drug combined with the LIVNS subgroup, and 15 were in the drug-only subgroup. The results of survival analysis indicated that the recurrence rate was lower in the drug + LIVNS subgroup in the vagal type (Figures 2,7). The analysis of Holter recordings was done every 3 months postoperatively in all patients. In the vagal type, there were 5 patients with PACs >2,000 beats/24 h in the drug subgroup 0 in the drug + LIVNS subgroup. In the common type, there were 11 in the drug subgroup and 6 in the drug + LIVNS subgroup. The contingency tables for the chi-square test results for vagal type and common type, respectively, showed that the number of frequent PACs in the drug + LIVNS subgroup of the vagal type was significantly lower than that in the drug subgroup (P<0.05) (Table 2).

Table 2
| Treatment method | LIVNS + drug | Drug | P value |
|---|---|---|---|
| Vagus type | 0.046 | ||
| PACs >2,000 beats/24 h | 0 | 5 | |
| PACs ≤2,000 beats/24 h | 18 | 20 | |
| N | 18 | 25 | |
| Common type | 0.88 | ||
| PACs >2,000 beats/24 h | 6 | 11 | |
| PACs ≤2,000 beats/24 h | 27 | 39 | |
| N | 33 | 50 |
PACs, premature atrial contractions; LIVNS, low-level vagus nerve stimulation.
Discussion
Previous literature suggests that dysfunction of the autonomic nervous system, including alterations in sympathetic and vagal tone as well as dysregulation, plays a crucial role in the initiation and maintenance of AF. In 1978, Coumel (1) demonstrated that stimulation of the cardiac autonomic nerves could induce AF in patients with typical cardiac conduction structures. Schauerte et al. (2) further elucidated that high-frequency stimulation of cardiac ganglia during the atrial refractory period could elicit rapid ectopic beats in the pulmonary veins and superior vena cava, subsequently triggering AF.
A quantitative study conducted by Chevalier et al. (3) on cardiac tissue histology from 43 adult autopsy cases revealed a higher density of nerves at the orifices of the four pulmonary veins compared to their distal ends. Notably, there was significantly greater innervation in the left upper pulmonary vein than in the right lower pulmonary vein, with minimal innervation observed in the endocardium. Furthermore, the atria exhibited a gradient distribution of nerve innervation, with more extensive innervation present in the left atrium and posterior wall relative to the right atrium and anterior wall. This heterogeneity in atrial nerve innervation may also contribute to AF induction via the autonomic nervous system.
According to Coumel (4,5), certain characteristic manifestations of AF may be mediated by vagal or sympathetic nerves, reflecting distinct neural influences on the condition. Moreover, electrical and structural remodeling during AF may affect the autonomic nervous system, resulting in “autonomic remodeling” and disrupting the balance between sympathetic and vagal tones. An imbalanced autonomic nervous system can facilitate the occurrence of AF, which in turn may lead to further remodeling of the autonomic system, promoting recurrent episodes of AF.
The cardiac autonomic nervous system has significant potential to influence and regulate the onset, maintenance, and termination of AF. Pappone et al. (6) introduced the concepts of vagal AF and sympathetic AF, highlighting the differing electrophysiological mechanisms and clinical characteristics associated with parasympathetic and sympathetic nervous system involvement in AF induction. Vagal AF often presents with characteristic clinical features during nighttime, at rest, and after meals—especially after dinner—when there is a gradual increase in vagal tone. Furthermore, radiofrequency ablation surgery has demonstrated superior outcomes in managing these AF types.
This study investigated vagal AF based on the patient responses during radiofrequency ablation. It performed analysis of the distribution characteristics of PACs at different time intervals based on Holter recording analysis. Patients with vagal-mediated AF showed a higher frequency of PACs during six particular periods: 0–1, 1–2, 12–13, 13–14, 19–20, and 20–21. These findings were in significant contrast to those of patients with non-vagal-mediated AF. A preliminary exploration of possible reasons is as follows: research (7) has shown that after eating, heart rate variability (HRV) typically increases, reflecting an elevation in vagal tone. The decrease in heart rate and the increase in HRV are associated with vagal nerve activation during the digestive process. Individuals who frequently experience PACs after meals often have increased vagal tone. From this perspective, the time periods of 12–13, 13–14, 19–20, and 20–21 coincide with the times after lunch and dinner. Additionally, vagal tone is also related to cortisol secretion; the levels of cortisol are at their lowest during the time periods of 0–1 and 1–2, while vagal tone tends to be higher (8). For example, patients with sick sinus syndrome often have an increased likelihood of sinus bradycardia or sinus arrest during these periods, while patients with vagally-mediated AF have an increased incidence of atrial arrhythmias. These observations provided valuable data for future research on using LIVNS for treating AF. Once complete electrical isolation of vagal AF was achieved, autonomic potentials from pulmonary veins could be recorded in 49% of the patients. Additionally, it was observed that the frequency of autonomic potentials in the pulmonary veins was mainly concentrated in the bilateral superior pulmonary veins (80%), which was attributed to the nerve fibers passing through the top of the left atrium, specifically the left atrial anterior ganglia plexus (VLAsGP), left posterior ganglia plexus (LDsGP), and middle posterior ganglia plexus (MDsGP) (9). The autonomic potentials in the pulmonary veins are caused by abnormal activity of the muscle cells or neural tissue in the pulmonary vein walls, which can trigger the onset of AF (10). AF-nests are generally considered to be sources of irregular excitations within the atria, and these irregular excitations are closely related to the autonomic potentials in the pulmonary veins (11). When the autonomic potentials in the pulmonary veins are excessively frequent or abnormal, they may lead to the triggering of AF. This study found that the vagally-mediated group had a higher likelihood of experiencing autonomic potentials following pulmonary vein isolation, mainly concentrated in the bilateral superior pulmonary veins. This may be because the superior pulmonary veins, which have a more extensive distribution of vagus nerve fibers, are subject to stimulation from the vagus nerve, enhancing the activity of autonomic potentials within the pulmonary veins. This mechanism is particularly prominent in vagally-mediated AF, further indicating a close relationship between autonomic potentials in the pulmonary veins and AF nests.
There has been an increased interest in using LIVNS as a treatment for AF. This approach has been reported to have significant clinical applications. The TREATAF (12) trial demonstrated that the AF burden can be decreased by 75% by non-invasive auricular vagus nerve stimulation. Stavrakis et al. (13) found that compared to the control group, the AF inducibility could be significantly reduced by providing 1 h of non-invasive vagus nerve stimulation prior to ablation surgery in patients with PAF. The patients were followed up after the surgery, and a subgroup of patients underwent LIVNS. A total of 43 vagal-type cases were identified after an average follow-up period of 666.72±275.40 days. In contrast, the follow-up period averaged 630.61±245.63 days for the 83 patients with the common type. Of these, 18 vagal-type cases (42%) and 33 common-type cases (40%) received LIVNS therapy. The survival analysis results indicate that when patients with a vagal type were treated with combined LIVNS therapy, they exhibited a reduced risk of AF recurrence compared to those receiving monotherapy. Moreover, as shown in Figure 7, the extent of risk reduction was more significant in vagal-type patients receiving combined LIVNS therapy than in common-type patients receiving combined LIVNS therapy. It is imperative to have at least one Holter that shows PACs exceeding 2,000 beats per day prior to enrolling patients. Following the surgery, a Holter monitoring should be done every 3 months. Compared to the subgroup receiving only drug therapy, the patient subgroup with the vagal type, who receive combined LIVNS, exhibit a lower risk of postoperative Holter-detected PACs exceeding 2,000 beats per day. This difference is statistically significant. However, as demonstrated in Table 2, this difference is not observed in patients with the common type. It is important to note that previous studies (14) have shown that the number of PACs is independently associated with the recurrence of AF. A cutoff of 1,431 PACs per 24 h is considered the optimal value for predicting AF recurrence. In this study, we referenced this literature and, combined with our clinical experience, chose a threshold of more than 2,000 PACs per day as an indicator for evaluating the risk of recurrence after radiofrequency ablation.
The aforementioned findings indicate that LIVNS might have a higher efficacy for patients with vagal type. The authors of this study believe that it is not easy to distinguish between vagal and non-vagal AF based solely on the clinical reactions of patients and the recorded changes in potentials during radiofrequency ablation surgery. However, grouping patients based on this method allows for observing distinct differences in the distribution characteristics of PACs in Holter, the occurrence of autonomous potentials after pulmonary vein isolation, and PACs occurrence and AF recurrence in the 72-h postoperative Holter for vagal type AF. Particularly, the follow-up results suggest that LIVNS is more effective in reducing the recurrence rate of AF and PACs, which can be distinguished as vagal types. Recent studies on AF pathogenesis have demonstrated that LIVNS can decrease the incidence of AF (15). This finding has also been validated in vivo studies (16) and clinical trials (17-20). Additionally, during cardiac surgery, the effect of LIVNS on cardiac conduction can be directly observed through patch electrodes (21). However, the pathogenesis of AF may not only be related to the vagus nerve but also involve myocardial ischemia (22), myocardial scar formation (23), rheumatic activity (24), viral infection (25), and other factors. Therefore, it is necessary to identify patients who are suitable for such treatment in clinical practice to enhance the efficacy of LIVNS. This requires an analysis of a series of clinical data or electrophysiological phenomena observed during surgery to find the patients whose AF is associated with the vagus nerve. This study is expected to help in achieving this objective. In patients with PAF, during surgery, AF can persist, and during the isolation of the pulmonary veins, high-frequency fractional potentials appear, followed by a transition to sinus rhythm, but at this point, the pulmonary veins have not been isolated. It cannot be definitively stated that observing this phenomenon is indicative of vagally mediated AF. What this study aims to demonstrate is that observing this phenomenon, along with adding low-intensity vagal nerve stimulation treatment postoperatively, will lead to a good prognosis. Additionally, it should be noted that during radiofrequency ablation, high-frequency fragmented potentials were observed at the catheter during persistent AF. The occurrence of a vagal response or transition to sinus rhythm after the ablation may also be a result of ablation reaching the AF-nests. The relationship between AF-nests and the vagus nerve is closely intertwined (26), and they play an important role in the pathogenesis of AF. Effective ablation of AF-nests may be one of the factors associated with better prognosis (27). In the cases of this study, regardless of whether low-intensity vagus nerve stimulation treatment was added postoperatively, the overall prognosis in the vagal type appeared to be better than that in the common type, although it did not reach statistical significance (Figure 7).
Limitations
The primary limitation of our study was that it was a single-center investigation. The criteria for identifying vagal-mediated AF may be subjective. Confirmation of this study’s results with a larger cohort of patients will be needed to confirm or reject our findings.
Conclusions
Vagal-mediated AF can be diagnosed based on a combination of symptoms, including nausea, vomiting, bradycardia, and the occurrence of an increase in the frequency of pulmonary vein potentials followed by a sudden restoration of sinus rhythm. Here, this type of AF exhibited a specific temporal pattern, with the highest frequency occurring at six specific time intervals: 0–1, 1–2, 12–13, 13–14, 19–20, and 20–21 h. Drug + LIVNS significantly reduced AF and PACs recurrence following catheter ablation for vagally mediated AF.
Acknowledgments
Funding: This work was supported in part by
Footnote
Data Sharing Statement: Available at https://jxym.amegroups.com/article/view/10.21037/jxym-24-27/dss
Peer Review File: Available at https://jxym.amegroups.com/article/view/10.21037/jxym-24-27/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jxym.amegroups.com/article/view/10.21037/jxym-24-27/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Dongguan Kanghua Hospital, Dongguan City, Guangdong Province, China (No. 2021024) and informed consent was obtained from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Coumel P, Attuel P, Lavallée J, et al. The atrial arrhythmia syndrome of vagal origin. Arch Mal Coeur Vaiss 1978;71:645-56. [PubMed]
- Schauerte P, Scherlag BJ, Patterson E, et al. Focal atrial fibrillation: experimental evidence for a pathophysiologic role of the autonomic nervous system. J Cardiovasc Electrophysiol 2001;12:592-9. [Crossref] [PubMed]
- Chevalier P, Tabib A, Meyronnet D, et al. Quantitative study of nerves of the human left atrium. Heart Rhythm 2005;2:518-22. [Crossref] [PubMed]
- Coumel P. Clinical approach to paroxysmal atrial fibrillation. Clin Cardiol 1990;13:209-12. [Crossref] [PubMed]
- Coumel P. Paroxysmal atrial fibrillation: role of autonomic nervous system. Arch Mal Coeur Vaiss 1994;87:55-62. [PubMed]
- Pappone C, Santinelli V, Manguso F, et al. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation 2004;109:327-34. [Crossref] [PubMed]
- Lu CL, Zou X, Orr WC, et al. Postprandial changes of sympathovagal balance measured by heart rate variability. Dig Dis Sci 1999;44:857-61. [Crossref] [PubMed]
- Fujiwara S, Shinkai S, Kurokawa Y, et al. The acute effects of experimental short-term evening and night shifts on human circadian rhythm: the oral temperature, heart rate, serum cortisol and urinary catecholamines levels. Int Arch Occup Environ Health 1992;63:409-18. [Crossref] [PubMed]
- Aksu T, Gupta D, Pauza DH. Anatomy and Physiology of Intrinsic Cardiac Autonomic Nervous System: Da Vinci Anatomy Card #2. JACC Case Rep 2021;3:625-9. [Crossref] [PubMed]
- Baykaner T, Rogers AJ, Meckler GL, et al. Clinical Implications of Ablation of Drivers for Atrial Fibrillation: A Systematic Review and Meta-Analysis. Circ Arrhythm Electrophysiol 2018;11:e006119. [Crossref] [PubMed]
- Arruda M, Natale A. Ablation of permanent AF: adjunctive strategies to pulmonary veins isolation: targeting AF NEST in sinus rhythm and CFAE in AF. J Interv Card Electrophysiol 2008;23:51-7. [Crossref] [PubMed]
- Stavrakis S, Stoner JA, Humphrey MB, et al. TREAT AF (Transcutaneous Electrical Vagus Nerve Stimulation to Suppress Atrial Fibrillation): A Randomized Clinical Trial. JACC Clin Electrophysiol 2020;6:282-91. [Crossref] [PubMed]
- Stavrakis S, Humphrey MB, Scherlag BJ, et al. Low-level transcutaneous electrical vagus nerve stimulation suppresses atrial fibrillation. J Am Coll Cardiol 2015;65:867-75. [Crossref] [PubMed]
- Fujisawa T, Kawakami H, Nagai T, et al. Premature atrial contraction immediately after catheter ablation was associated with late recurrence of atrial fibrillation. Pacing Clin Electrophysiol 2023;46:152-60. [Crossref] [PubMed]
- Li S, Scherlag BJ, Yu L, et al. Low-level vagosympathetic stimulation: a paradox and potential new modality for the treatment of focal atrial fibrillation. Circ Arrhythm Electrophysiol 2009;2:645-51. [Crossref] [PubMed]
- Liu F, Sun W, Li Y, et al. Low-Level Stimulation and Ethanol Ablation of the Vein of Marshall Prevent the Vagal-Mediated AF. Front Cardiovasc Med 2021;8:675485. [Crossref] [PubMed]
- Yu L, Scherlag BJ, Li S, et al. Low-level vagosympathetic nerve stimulation inhibits atrial fibrillation inducibility: direct evidence by neural recordings from intrinsic cardiac ganglia. J Cardiovasc Electrophysiol 2011;22:455-63. [Crossref] [PubMed]
- Sheng X, Scherlag BJ, Yu L, et al. Prevention and reversal of atrial fibrillation inducibility and autonomic remodeling by low-level vagosympathetic nerve stimulation. J Am Coll Cardiol 2011;57:563-71. [Crossref] [PubMed]
- Sha Y, Scherlag BJ, Yu L, et al. Low-level right vagal stimulation: anticholinergic and antiadrenergic effects. J Cardiovasc Electrophysiol 2011;22:1147-53. [Crossref] [PubMed]
- Kharbanda RK, van der Does WFB, van Staveren LN, et al. Vagus Nerve Stimulation and Atrial Fibrillation: Revealing the Paradox. Neuromodulation 2022;25:356-65. [Crossref] [PubMed]
- Kharbanda RK, Ramdat Misier NL, van Schie MS, et al. Insights Into the Effects of Low-Level Vagus Nerve Stimulation on Atrial Electrophysiology: Towards Patient-Tailored Cardiac Neuromodulation. JACC Clin Electrophysiol 2023;9:1843-53. [Crossref] [PubMed]
- Gaudino M, Di Franco A, Rong LQ, et al. Postoperative atrial fibrillation: from mechanisms to treatment. Eur Heart J 2023;44:1020-39. [Crossref] [PubMed]
- Mannion J, Galvin J, Boles U. Left atrial scar identification and quantification in sinus rhythm and atrial fibrillation. J Arrhythm 2020;36:967-73. [Crossref] [PubMed]
- Ciconte G, Conti M, Evangelista M, et al. Atrial Fibrillation in Autoimmune Rheumatic Diseases: from Pathogenesis to Treatment. Rev Recent Clin Trials 2018;13:170-5. [Crossref] [PubMed]
- Manolis AS, Manolis AA, Manolis TA, et al. COVID-19 infection and cardiac arrhythmias. Trends Cardiovasc Med 2020;30:451-60. [Crossref] [PubMed]
- Oh S, Kong HJ, Choi EK, et al. Complex fractionated electrograms and AF nests in vagally mediated atrial fibrillation. Pacing Clin Electrophysiol 2010;33:1497-503. [Crossref] [PubMed]
- Mateos JC, Mateos EI, Lobo TJ, et al. Radiofrequency catheter ablation of atrial fibrillation guided by spectral mapping of atrial fibrillation nests in sinus rhythm. Arq Bras Cardiol 2007;89:124-34, 140-50. [PubMed]
Cite this article as: Chen C, Zhang J, Li Q, Fu Y, Tu L, Li G. The Holter characteristics of vagal-mediated paroxysmal atrial fibrillation and clinical observation of low-level vagus nerve stimulation after radiofrequency ablation. J Xiangya Med 2024;9:14.


