
Dystrophin Dp71, a novel tumor suppressor?
Dystrophins belong to a group of proteins encoding by the Duchenne muscular dystrophy (DMD) gene. DMD gene generates full-length dystrophin (427 kDa) and different C-terminal truncated isoforms through alternative usage of internal promoters. Short isoforms are termed in accordance with their molecular mass as Dp140, Dp116, Dp71 and Dp40. Dystrophin Dp71, is ubiquitously expresses, with the exception of skeletal muscle, and is the predominant DMD gene product in nervous system. In addition, Dp71 undergoes alternative splicing of exons 71 and/or 78 to generate different splicing variants, being Dp71f (−71/−78) and Dp71d (−71/+78) the most studied isoforms. While deficiency of dystrophin causes muscle degeneration, absence of Dp71 has been implicated in cognitive impairment and abnormal retinal physiology, two well-defined non-muscular alterations of DMD patients, reviewed in (1). As dystrophin does, Dp71 associates with sarcoglycans, dystroglycans, syntrophins and dystrobrevin, to conform the plasma membrane-associated dystrophin associated protein complex. Such protein assembly provides stability to the plasma membrane and modulates cell signaling. Interestingly, Dp71 undergoes nuclear import trough recognition of an atypical nuclear localization signal by the importin α2/β1 system. Nuclear Dp71 in turn, is involved in the maintenance of nuclear architecture, via its interaction with nuclear envelope proteins, including emerin and lamins A/C and B1 (2). Thus, it appears that Dp71 is a multifunctional protein that interacts with different partners in both the cytoplasm and nucleus, which enables it to modulate a variety of cell functions.
Unexpectedly, the study published in Oncotarget [2016] by the Tan and Xiao group implicates Dp71 for the first time in cancer development (3). The authors performed a combinatory study analyzing both clinical samples from gastric cancer patients and different gastric cancer cell lines. Remarkable, western blot analysis of gastric adenocarcinoma samples showed clear reduction of Dp71 expression, compared with adjacent non-tumor samples. Furthermore, immunohistochemistry analysis of cancer samples revealed decreased labeling of Dp71 in most of the clinical samples; interestingly, Dp71 downregulation appears to be associated with gastric cancer differentiation, being the most poorly differentiated tumor samples which exhibited the lowest levels of Dp71. Consistent with this, Dp71 deficiency correlates with poor survival of patients after tumor surgical resection. Supporting clinical findings, Dp71 expression was found downregulated in different gastric cancer cell lines, compared with a control gastric epithelial cell line. Overall these findings point to Dp71 as a key protein for the maintenance of cellular homeostasis, in such a way that Dp71 deficiency might alter some basic cellular functions, which dysregulation are related to cancer progression, namely cell cycle progression, cell migration, invasion, anchorage independence and/or invadopodia formation. Consistent with a role for Dp71 as tumor suppressor, the authors showed that forced expression of Dp71 resulted in proliferation inhibition of gastric cells.
Although this study opens a new exciting avenue in the biology of Dp71, elucidation of molecular mechanisms underlying its implication in cancer clearly requires further investigation. In this scenario, two immediate important questions arise: how Dp71 expression declines in a cancerous environment? And how Dp71 deficiency alters cancer-associated cellular functions. With respect to the first question, Tan et al. suggest that Dp71 expression is subjected to negative regulation at transcriptional level in the cancerous gastric epithelium, because two transcription factors that positively regulates Dp71 promoter (HNF3α and KLF4) were found downregulated in gastric cancer. Alternatively, since DMD genetic alteration has been found in a significant percentage of non-myogenic tumors (4), it is possible that disturbance of Dp71 expression is caused by mutations located within its coding sequence. The second question is definitely more complex to approach; however, based on a previous key study (5), the authors envisaged that Dp71 deficiency might impair lamin B1 expression/function, which in turn might favor cancer progression. Lamin B1 is a nuclear envelope protein involved in crucial nuclear processes, including nuclear morphology, heterochromatin organization, cell division and senescence. Villarreal et al. (5) established the association of Dp71 with lamin B1 in PC12 cell nuclei and importantly demonstrated that this interaction is relevant for lamin B1 stability and consequently for cell proliferation. Following this clue, the authors confirmed Dp71-lamin B1 interaction now in gastric cells; subsequently, they revealed decreased levels of lamin B1 in both gastric tumor samples and gastric cancer cells. Furthermore, Tan et al. showed that lamin B1 overexpression inhibited proliferation of gastric cells. Overall these data suggest that Dp71-lamin B1 association is physiological relevant for regulating gastric cell division and that disruption of this interaction is a necessary step for tumor development. Since Dp71 colocalizes with lamin B1 at the mitotic spindle (5), the authors claimed that concurrent deficiency of both Dp71 and lamin B1 can alter mitosis progression, which ultimately might lead to chromosomal instability (Figure 1), a hallmark of cancer cells. In addition, association of lamin B1 foci with major nuclear compartments (chromatin, nucleoli and nuclear speckles) during mitosis serves as landmark to guide post-mitotic nuclear reassembly (6). It is worth to note that Dp71 is able to interact with additional nuclear proteins, to conform alternative nuclear protein assemblies. Dp71 interacts with emerin and lamina AC to constitute a nuclear envelope protein complex involved in the maintenance of nuclear structure (1). In addition, Dp71 associates with several dystrophin associated proteins to constitute a nuclear DAPC (1). Then, decreased expression of Dp71 observed in gastric cancer cells might disrupt these protein assemblies, with predictable physiological consequences. In fact, aberrant nuclear morphology, accompanied with decreased levels of emerin and lamin A/C has been reported in certain types of cancer.
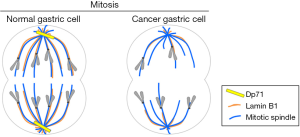
All the evidences described above considered only the nuclear localization of Dp71; however, a failure in Dp71 function can also impact the plasma membrane or cytoplasm. Since Dp71f isoform has been implicated with the β1-integrin adhesion apparatus (1), its deficiency might alter the migratory and invasive capacities of tumor cells.
In summary, the study by Tan et al. identified an unrecognized role for Dp71 as tumor suppressor protein (3). Experimental modulation of Dp71 expression in cancer models and a better characterization of the Dp7-lamin B1 interaction will help to decipher the participation of Dp71 in cancer, as well as to sustain its applicability for cancer prognosis/therapy.
Acknowledgments
Funding: Contract grant sponsors: CONACyT (Mexico); Contract grant numbers: 258268 to RSS and 237123 to BC.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Xiangya Medicine. The article did not undergo external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2016.12.13). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tadayoni R, Rendon A, Soria-Jasso LE, et al. Dystrophin Dp71: the smallest but multifunctional product of the Duchenne muscular dystrophy gene. Mol Neurobiol 2012;45:43-60. [Crossref] [PubMed]
- Suárez-Sánchez R, Aguilar A, Wagstaff KM, et al. Nucleocytoplasmic shuttling of the Duchenne muscular dystrophy gene product dystrophin Dp71d is dependent on the importin α/β and CRM1 nuclear transporters and microtubule motor dynein. Biochim Biophys Acta 2014;1843:985-1001.
- Tan S, Tan J, Tan S, et al. Decreased Dp71 expression is associated with gastric adenocarcinoma prognosis. Oncotarget 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Luce LN, Abbate M, Cotignola J, et al. Non-myogenic tumors display altered expression of dystrophin (DMD) and a high frequency of genetic alterations. Oncotarget 2016; [Epub ahead of print]. [PubMed]
- Villarreal-Silva M, Centeno-Cruz F, Suárez-Sánchez R, et al. Knockdown of dystrophin Dp71 impairs PC12 cells cycle: localization in the spindle and cytokinesis structures implies a role for Dp71 in cell division. PLoS One 2011;6:e23504. [Crossref] [PubMed]
- Martin C, Chen S, Jackson DA. Inheriting nuclear organization: can nuclear lamins impart spatial memory during post-mitotic nuclear assembly? Chromosome Res 2010;18:525-41. [Crossref] [PubMed]
Cite this article as: Suárez-Sánchez R, Cisneros B. Dystrophin Dp71, a novel tumor suppressor? J Xiangya Med 2016;1:13.


