
Cryoglobulinemia complicated with multi-focal serous cavity hydrops: a case report and literature review
Introduction
An immunoglobulin, characterized by pathological in vitro precipitation at temperatures less than 37 °C and redissolving after rewarming, was firstly described in a patient with multiple myeloma in 1933. This kind of immunoglobulin was termed cryoglobulin in 1947. Brouet et al. classified cryoglobulin into three basic types according to the clonality and composition of immunoglobulins (1). Most cases of cryoglobulinemia have primary causes, of which hepatitis C virus (HCV) infection is the most common. Cryoglobulin produce organ damage through two main pathways: vascular sludging and immune-mediated mechanisms. The most frequently affected internal organs are the peripheral nerves, kidneys, and joints. The main symptom of cryoglobulin vasculitis is purpura (2).
Case presentation
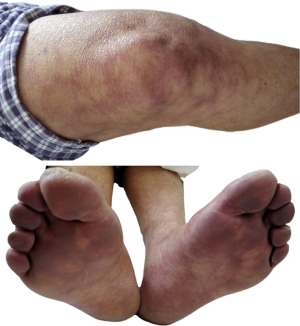
The patient was a 75-year-old male with a previous history of chronic obstructive pulmonary disease (COPD) for more than 10 years. Chronic symptoms of coughing and expectoration were aggravated 10 days ago prior to eventual admission to our department of respiratory disease. His medical history was notable for repeated hospitalizations at other hospitals for chronic cough, skin cyanosis, visible subcutaneous blood vessels (Figure 1) and arthralgia with colder temperatures. These symptoms were present for more than 7 years. Over the last 6 years, he was repeatedly admitted for multi-focal serous cavity hydrops. Rapid formation of precipitates in blood samples was noted during these admissions. His physicians could not identify an etiology for the recurrent, multi-focal serous cavity hydrops precipitated by cold exposure, nor establish a relationship to his respiratory symptoms. He therefore followed a standard treatment regimen for COPD.
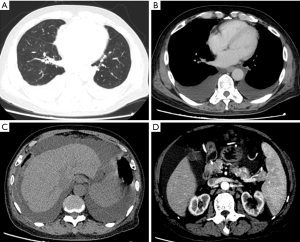
After admission to our center, standard treatment for COPD was intensified for symptomatic relief. At the same time a diagnostic workup was initiated with the goal to identify a potential unifying etiology for his symptoms. Routine blood tests were unremarkable except for moderate anemia. Blood biochemistry tests reported a slight decline of albumin (37.9 g/L), with normal liver function tests. HCV, human immunodeficiency virus (HIV), hepatitis B surface antigen (HbsAg), and treponema pallidum (TP) infections were all negative. Rheumatological disorder related test identified an elevated serum level of IgM, low level of complement C3 and C4 and a slightly elevated rheumatoid factor (RF). Anti-human globulin test (Coomb’s test) was positive. Cryoglobulin test was positive (Figure 2). Serum and urinary light chain were in the normal range. Uric Bence Jones protein was positive. Bone marrow biopsy showed lymphocytes and plasma cells in the bone marrow. Flow cytometry of bone marrow reported that 14.4% cells with the phenotype considered being monoclonal B cells (CD19+, CD20+). Serum immunofixation electrophoresis detected an M protein band and an anti-IgM band (Figure 3). The bone marrow smears were of poor quality. Diagnostic imaging included normal echocardiography and a computed tomography (CT) scan of the chest and abdomen. The CT of the chest was positive for large bilateral pleural effusion and typical changes of COPD (Figure 4), multifocal enlarged lymph nodes (bilateral neck, axillae, mediastinal and retroperitoneal). The CT of the abdomen was remarkable for thickening of the greater omentum, an enlarged spleen, and ascites, a paracentesis and thoracocentesis was performed and chest tube drainage was inserted. The chest tube drained 2 liters of light yellow coloured fluid. Routine and biochemical assay of pleural fluid and ascitic fluid were consistent with transudate according to Lights Standard (3).

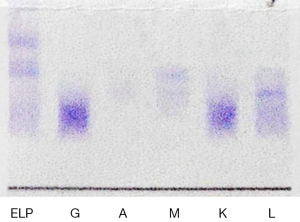
A diagnosis of Waldenström’s macroglobulinemia (WM), with the complications of cryoglobulinemia and multiple serous cavity hydrops was established. The symptoms of the patient were relieved with intensified medical management and drainage of pleural effusions and ascites. At that time the patient requested to be discharged against medical advice and refused subsequent treatment. Unfortunately, he did not follow-up for further management at our center.
Discussion
Cryoglobulins are generated by the clonal expansion of B cells, in the context of either lymphoproliferative disorders or persistent immune stimulation triggered by chronic infections or autoimmune diseases (4). Many illnesses, including infections, autoimmune disorders and malignancies, are associated with cryoglobulinemia (5); the most common cause is HCV infection, and nearly 90% of patients with mixed cryoglobulinemia have detectable circulating HCV-RNA (6). Cryoglobulins produce organ damage mainly through two mechanisms: one is vascular sludging results from cryoglobulin precipitation in the microcirculation, mainly in type I cryoglobulinemia; the other is immune-mediated mechanism, principally vasculitis, occur mainly in mixed cryoglobulinemia (7). The diagnosis of cryoglobulinemia requires knowledge of the condition and heightened suspicion by the clinician. 2–50% of people with cryoglobulin in the serum are symptomatic. Cryoglobulinemia leads to variable symptoms including characteristic purpura, ischemia of the extremities, renal failure, peripheral neuropathy, abdominal pain secondary to intestinal ischemia and arthralgias (8). Therefore cryoglobulin testing is of great importance in general clinical practice (2). The prognosis is associated with the severity of organ damage and the primary underlying disease. More than 90% cryoglobulinemia has an underlying cause; thus, the treatment of cryoglobulinemia mainly aims to treat the primary disease.
Cryoglobulinemia can be the presentation of WM (9). WM is a lymphoproliferative disorder characterized primarily by bone marrow infiltration and IgM monoclonal gammopathy. The clinical manifestations of WM are non-specific. Anemia, thrombocytopenia, hepatosplenomegaly, lymph node enlargement, and pleural effusions are found. Presence of IgM monoclonal protein associated with ≥10% clonal lymphoplasmacytic cells in bone marrow confirms the diagnosis (10).
According to the clinical manifestations and the cryoglobulin test, the diagnosis of cryoglobulins was confirmed in our patient. The primary cause of the cryoglobulinemia in this case was considered to be WM, which were supported by the following findings: anemia and splenomegaly; an increased level of circulation IgM; bone marrow biopsy showed the lymphocytes infiltration; flow cytometry of bone marrow confirm the immunophenotype to be monoclonal B cells (≥10%) (Table 1). The multi-focal serous cavity effusions found in our patient have not been reported in this context, and its pathophysiologic etiology remained unclear. We excluded right heart failure and hypoproteinemia. A potential explanation is the presence of malignant hyperplastic pleural infiltrates of atypical lymphocytes, which could lead to the damage of the capillaries and thus increased permeability.
Table 1
| Items | Results |
|---|---|
| Routine blood tests | WBC 5.3×109/L; RBC 2.48×1012/L; hemoglobin 91 g/L; platelet count 201×109/L; hematocrit 26.5%; MCV 106.7 fl; MCH 36.6 PG. |
| Rheumatological evaluation | IgM: 2,890 (range, 630–2,770) mg/L, IgG: 9.15 (range, 7.23–16.85) g/L, IgA: 471.00 (range, 690–3,820) mg/L, C4: 31.50 (range, 120–360) mg/L, C3: 373.00 (range, 850–1,930) mg/L, RF: 36.10 (range, 0–30) IU/mL* |
| Flow cytometry of bone marrow | CD45st+, CD19+, CD5dim+, CD23-, FMC7+, CD20+, CD22+, sλ+, sκ– (account for14.4%) |
| Bone marrow biopsy | Lymphocytes and plasma cells could be found in the bone marrow |
| Cryoglobulin test | Positive |
| Serum immunofixation electrophoresis | M protein band and anti-IgM band |
*, reference values are shown in brackets. MCV, mean corpuscular volume; MCH, mean cell hemoglobin.
Conclusions
Although multiple serous cavity hydrops could be triggered by multiple causes, intractable and recurrent serous cavity hydrops deserve more attention, especially in the context of unclear, complex clinical scenarios. A comprehensive evaluation for underlying causes should be considered in order to avoid misdiagnosis. Moreover, even though HCV infection is the predominate cause of cryoglobulinemia, other etiology diagnosis should be taken into consideration when HCV is negative.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2017.02.12). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brouet JC, Clauvel JP, Danon F, et al. Biologic and clinical significance of cryoglobulins. A report of 86 cases. Am J Med 1974;57:775-88. [Crossref] [PubMed]
- Ramos-Casals M, Stone JH, Cid MC, et al. The cryoglobulinaemias. Lancet 2012;379:348-60. [Crossref] [PubMed]
- Light RW, Macgregor I, Ball WC, Luchsinger PC. Pleural-Fluid Lactic Acid Dehydrogenase and Protein Content. Ann Intern Med. 1972;880. [Crossref]
- Sargur R, White P, Egner W. Cryoglobulin evaluation: best practice? Ann Clin Biochem 2010;47:8-16. [Crossref] [PubMed]
- Dammacco F, Lauletta G, Montrone M, et al. Mixed cryoglobulinemia: a model of virus-related disease in internal medicine. Dig Liver Dis 2007;39:S8-S12. [Crossref] [PubMed]
- Ferri C, Greco F, Longombardo G, et al. Association between hepatitis C virus and mixed cryoglobulinemia Clin Exp Rheumatol 1991;9:621-4. [see comment]. [PubMed]
- Sansonno D, Tucci FA, Ghebrehiwet B, et al. Role of the receptor for the globular domain of C1q protein in the pathogenesis of hepatitis C virus-related cryoglobulin vascular damage. J Immunol 2009;183:6013-20. [Crossref] [PubMed]
- Chan AO, Lau JS, Chan CH, et al. Cryoglobulinaemia: clinical and laboratory perspectives. Hong Kong Med J 2008;14:55-9. [PubMed]
- Michael AB, Lawes M, Kamalarajan M, et al. Cryoglobulinaemia as an acute presentation of Waldenstrom's macroglobulinaemia. Br J Haematol 2004;124:565. [Crossref] [PubMed]
- Gertz MA. Waldenström macroglobulinemia: 2012 update on diagnosis, risk stratification, and management. Am J Hematol 2012;87:503-10. [Crossref] [PubMed]
Cite this article as: Zhao L, Xiao Q, Hu C, Tang Y, Qin L. Cryoglobulinemia complicated with multi-focal serous cavity hydrops: a case report and literature review. J Xiangya Med 2017;2:21.


