Developing an evidence-based clinical algorithm for the assessment, diagnosis and management of acute subarachnoid hemorrhage: a review of literature
Introduction
Subarachnoid hemorrhage (SAH) is a neurologic emergency with a mortality rate of about 60% in the first 6 months. It occurs when blood is present in the subarachnoid space due to rupture of an intracranial blood vessel. Patients with uncontrolled hypertension, with history of smoking and excessive alcohol intake are found to have higher risk for its disabling occurrence (1). In a systematic review on the worldwide incidence of SAH, the overall incidence is 9 per 100,000 person-years with rates higher in Japan and Finland (2). In the Philippines, no local epidemiology data was found. International guidelines are only available in Europe and United States hence it is just prudent to formulate an evidence-based clinical algorithm in the local standpoint (3).
Objectives
The objectives of this study are to conduct a review of literature on the current relevant literature on the assessment, diagnosis and management of SAH and to develop an evidence-based clinical algorithm for acute SAH applicable on the local setting.
Methodology
Search strategy
A comprehensive review of the most relevant SAH literature published between 1985 and 2015 was undertaken in the process of developing the clinical algorithm. The search strategy was designed to identify relevant literature that focused on the assessment, diagnosis and management of acute SAH. Electronic databases (Medline, PubMed, Google Scholar and Herdin) were searched. The inclusion criteria included papers published after 1985, written in English and only focusing on acute SAH.
Study selection and quality of assessment
All titles and abstracts retrieved by the initial search were scanned using the following screening question: “Does the article discuss the clinical assessment, diagnosis and management of acute SAH?” If the article was deemed to meet the screening question, the full text was retrieved for assessment and review for the construction of the clinical algorithm. The level of evidence was assigned according to the recommendations of the National Guideline Clearinghouse (Table 1).
Table 1
| IA | Evidence from meta-analysis of randomized controlled trials |
|---|---|
| IB | Evidence from at least one randomized controlled trial |
| IIA | Evidence from at least one controlled study without randomization |
| IIB | Evidence from at least one other type of quasi-experimental study |
| III | Evidence from non-experimental descriptive studies, such as comparative studies, correlation studies, and case-control studies |
| IV | Evidence from expert committee reports or opinions or clinical experience of respected authorities, or both |
Data extraction
Articles deemed relevant were then used to construct the clinical algorithm. In an attempt to aid clinical management, the flow of the algorithm was developed with consideration of the current local practice on the assessment, diagnosis and management of SAH. Recommendations from the neurologists and neurosurgeons from the Philippine General Hospital-Department of Neurosciences were taken into account and discussed upon for consensus in one of the regular conference meetings of the Department.
Results
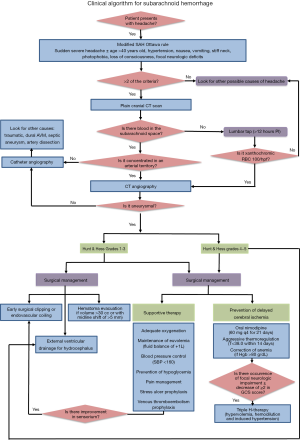
A total of 345 articles were retrieved and 40 of them were deemed relevant and reviewed to construct the SAH clinical algorithm (Table S1). Table 2 summarizes the level of evidence of the included studies. The clinical algorithm is divided into three key phases of acute SAH that have been addressed by the included literature. These phases are (I) Assessment, (II) Diagnosis and (III) Management. The SAH clinical algorithm developed is shown in Figure 1.
Table 2
| Level of evidence | Included articles (%) |
|---|---|
| I | 7 (17.5) |
| II | 3 (7.5) |
| III | 22 (55.0) |
| IV | 8 (20.0) |
Assessment
Symptom of severe headache (level of evidence = III and IV)
The clinical presentation of SAH is one of the most distinctive in medicine with the hallmark of “the worst headache of my life”, which is described by 80% of patients who can give a history. The headache is characterized as being extremely sudden and immediately reaching maximal intensity known as “thunderclap headache” (4). In a prospective study of 102 patients presenting with sudden headache, 50% had acute SAH and the headache progressed within five minutes in 19% of the patients (5). It may develop in any location, may be localized or generalized, may resolve spontaneously or may be relieved with analgesics (6).
Other associated clinical findings (level of evidence = IIb, III and IV)
The onset of headache may be associated with additional signs and symptoms, including nausea and/or vomiting, stiff neck, photophobia, brief loss of consciousness, or focal neurological deficits including cranial nerve palsies (7). Patients may report symptoms before the major rupture known as the “sentinel bleed” or “warning leak” occurring within 8 weeks before the overt SAH, hence a strong index of suspicion may be life-saving (8). In a study of 109 patients with SAH, 74% of patients presented with headache, 77% with nausea and vomiting, 53% with loss of consciousness and 35% with nuchal rigidity (7). In another study of 1,076 patients, 166 (15.4%) of patients presented with sudden episode of headache, vomiting, nuchal pain, dizziness and drowsiness (9).
Despite the classic presentation of SAH, ~6% of patients were misdiagnosed which may be fatal (10). If only headache is present, the chance of acute SAH being the cause is only 10% (7). In the Ottawa SAH rule, the presentation of more than one of the following in addition to headache (age ≥40 years old, neck pain/stiffness, witnessed loss of consciousness, onset during exertion, and limited neck flexion) is ~100% sensitive in making clinical decision for further investigation (11). This was chosen to be the basis of the clinical pathway with the addition of hypertension in the list as agreed upon in the consensus conference, especially that SAH occurs 15-fold in hypertensive patients (12).
Diagnosis
Cranial computed tomography scan (level of evidence = IIA and III)
A non-contrast cranial CT scan remains to be the cornerstone of diagnosis of SAH with a sensitivity of close to 100% in the first 3 days after which it decreases moderately during the next few days because of clearing from the spontaneous lysis of the subarachnoid blood (13). In a study of 3,521 patients with acute SAH who underwent plain cranial CT scanning, 92% were positive on the day of rupture but declined to 86% on the day after, 76% 2 days later and 58% 5 days later (14). In another recent study with 953 patients who underwent CT scanning within 6 hours of onset of severe headache, results yielded 100% sensitivity and specificity in identifying SAH (15). The pattern of hemorrhage may often suggest the cause of SAH. Aneurysmal rupture causing 85% of SAH is characterized by blood confined in the cisterns while traumatic cause present in 10% of SAH is characterized by blood confined in the superficial sulci and convexity of the brain (16).
lumbar tap (level of evidence = III)
After 5 days, the rate of negative CT increases sharply, and lumbar puncture is often required, though not usually followed in practice (17,18). The findings of a lumbar puncture indicating SAH include an elevated opening pressure, an elevated red blood cell count and the presence of xanthochromia in cerebrospinal fluid which can only be detected reliably 12 hours after the hemorrhage (19). In a study of 111 patients with SAH who underwent lumbar puncture between 12 hours and 2 weeks, all have xanthochromic cerebrospinal fluid (20).
CT angiography (CTA) (level of evidence = III)
CTA with a 64-slice scan, on the other hand, is an accurate tool for detecting and characterizing aneurysms, and also in deciding whether coiling or clipping should be done. In one series, the cause of SAH was detected with CTA in 62 out of the 65 patients with a 94% sensitivity and 100% specificity and it revealed the aneurysm in 46 of 47 patients with a sensitivity of 98% and a specificity of 100% (21). In another study, CTA was found to be 96.4% sensitivity and 96% specificity (22).
Catheter angiography (level of evidence = III)
In negative CTA or diffuse SAH, catheter angiography is indicated. If it turns out to be negative initially, a repeat catheter angiography is valuable after 7 days (23).
Management
SAH grading (level of evidence = IV)
The Hunt and Hess scale, being one of the most utilized grading systems in SAH, is based upon a five-point scale, where grade 5 is most severe (24). Hunt and Hess grades 1–3 carry a favorable prognosis and generally warrant aggressive treatment while Hunt and Hess grade 4–5 have 60–100% mortality rate, putting surgery less beneficial (25). The World Federation of Neurological Surgeons scale is another popular five-grade scale commonly used in clinical practice and has replaced the Hunt and Hess scale at many institutions (26). However, other factors such as older age, preexisting medical condition, hyperglycemia, sepsis, fever, delayed cerebral ischemia (DCI) and rebleeding carries a poorer prognosis (3).
Early surgery (level of evidence = IB, IIB, III and IV)
More than 1/3 of rebleeding occur within 3 hours and nearly half within 6 hours of symptom onset and early rebleeding is associated with worse outcome than later rebleeding (27). Surgical clipping or endovascular coiling of the ruptured aneurysm should be performed as early as feasible in the majority of patients to reduce the rate of rebleeding. In the International Subarachnoid Aneurysm Trial, an improved 1-year clinical outcome for patients with ruptured intracranial aneurysms treated with endovascular coiling compared to surgical clipping was observed, though the results are not generalizable because the trial only involved good-grade aneurysms. Moreover, the odds ratio for poor outcome at 2 months were 1.16 (95% CI, 0.89–1.50) for treatment (clipping and coiling combined) at 3–4 days, 1.39 (95% CI, 1.08–1.80) for treatment at 5–10 days, and 1.84 (95% CI, 1.36–2.51) for treatment after 10 days. The risk for poor outcome was highest when treatment was performed after day 10 hence postponing treatment in patients who are eligible for treatment between days 5–10 after SAH is not recommended (28). Patients presenting with vasospasm may be better treated with endovascular techniques (29). Antifibrinolytic therapy has been shown to reduce the incidence of aneurysm rebleeding when there is a delay in aneurysm obliteration but does not improve the patient’s outcome (30).
In a retrospective review, aneurysm repair immediately followed by hematoma evacuation for intracerebral hemorrhage greater than 30 cc or midline shift greater than 5 mm was associated with faster time to aneurysm protection, decreased length of hospital stay and cost (31). In another study, hematoma evacuation within 3.5 hours among patients with intraparenchymal hematoma greater than 50 mL has been shown to improve outcome (32).
Acute hydrocephalus, occurring in about 20–30% of patients within 72 hours is caused by obstruction of the CSF flow in the tentorial hiatus by extensive hemorrhage in the perimesencephalic cisterns, or in the ventricles by frank intraventricular hemorrhage (33,34). Acute hydrocephalus associated with SAH is usually managed preferably by external ventricular drainage (EVD) but may also do lumbar drainage. EVD for patients with SAH-associated hydrocephalus is generally associated with neurological improvement (35). In a randomized, controlled trial, gradual multistep EVD weaning that takes place over a 96-hour period provided no advantage to patients with aneurysmal SAH in preventing the need for long-term shunt placement and prolonged hospital stays compared to rapid weaning (36).
Prevention of DCI (level of evidence = IA, IB, III and IV)
DCI which involves vasospasm of the large arteries is one of the most common causes of death and disability in SAH most frequently occurring 7–10 days after aneurysm rupture and resolving spontaneously after 21 days (37). DCI is currently defined as the occurrence of focal neurological impairment such as hemiparesis, aphasia, apraxia or a decrease of at least two points on the Glascow Coma scale either on the total score or on one of the components which should last for at least 1 hour and not attributed to other causes by means of clinical assessment (38). In a retrospective cohort study involving 271 patients with SAH from a ruptured cerebral aneurysm, the modified Fisher scale was used to assess the admission CT scan and it was found that the risk of delayed cerebral ischemia increased with increasing grade (39). Table 3 shows the Modified Fisher grading with the risk for DCI. Despite extensive research into pharmacological treatment of DCI, the L-type dihydropyridine calcium channel antagonist Nimodipine remains the only intervention to consistently reduce the incidence of DCI and improve outcomes after SAH by preventing large artery narrowing (40). In the British Aneurysm Nimodipine Trial which is the largest study on this drug, oral Nimodipine 60 mg given every 4 hours after 21 days of SAH showed significant reductions in both the incidence of cerebral infarction and poor neurological outcomes at 3-months post-SAH (41). Another means for prevention of DCI is by aggressive thermoregulation. In one study, fever with a temperature greater than 38.3 occurs in as high as 70% of SAH patients within the first week and it have been associated with higher risk of symptomatic cerebral vasospasm (42). In another study, fever within 14 days of SAH with a mean body temperature of more than 38.0 °C in 2 consecutive days is associated with poor outcome, though it should be considered that intraventricular and extravasated blood may contribute to the hyperthermia (43). The treatment of anemia in SAH remains a very active area of investigation since it has been shown in a small group of patients with SAH that transfusion of a single unit of packed red blood cell led to increased cerebral oxygen delivery in all brain regions except those in the territory of vasospasm hence the need to prevent it by using packed red blood cell transfusions as needed to maintain a goal hemoglobin concentration of 8 g/dL (44).
Table 3
| Modified Fisher Score | CT scan findings | Risk for DCI (%) |
|---|---|---|
| 0 | No SAH or IVH | 0 |
| 1 | Minimal/thin SAH, no IVH | 6 |
| 2 | Minimal/thin SAH, with IVH in both lateral ventricles | 15 |
| 3 | Dense SAH, no IVH | 35 |
| 4 | Dense SAH, with IVH in both ventricles | 34 |
IVH, intraventricular hemorrhage; SAH, subarachnoid hemorrhage.
Triple-H therapy (level of evidence = IB)
Once DCI is evident, Triple-H therapy may be instituted. Triple-H therapy consisting of hypervolemia, hemodilution, and hypertension aim to increase cerebral perfusion in SAH patients with DCI, however, there is no consensus yet on how Triple-H or its separate component should be applied. Hypertension seems to be more effective in increasing cerebral blood flow than hemodilution or hypervolemia. However, even if induced hypertension is the most effective among the Triple-H therapy, its efficacy in reducing DCI is based on case series only, and not on a randomized clinical trial. This was the rationale behind the HIMALAIA Trial, a multicentre randomized controlled trial started in 2010 to investigate the outcome after induced hypertension versus no induced hypertension in patients with DCI after SAH, and to assess whether induced hypertension results in improved cerebral blood flow as measured by means of perfusion-CT however, results are yet to be published (45). To date, no randomized trials of the Triple-H therapy have been completed but the rapid improvement of many patients with this therapy and their worsening when it is stopped prematurely are convincing proof of efficacy. The exact mechanism of benefit is unclear (46). In a Systematic Review on Triple-H therapy, hemodilution is achieved by infusion of 70% dextran and 4% albumin while hypervolemia is commonly achieved by infusion of 4–5% albumin solution with the total volume of administered fluids varying between 250–4,000 mL per day. To induce hypertension either phenylephrine or dopamine was used in most studies resulting in an average increase in mean arterial pressure of 21–33 mmHg (47).
Supportive therapy (level of evidence = IB, III and IV)
The initial evaluation of patients with SAH should focus on airway evaluation. If the patient is lethargic or agitated, intubation should be immediately considered. It is also important to aim for euvolemia and avoid prophylactic hypervolemic therapy because there is evidence for harm and rebleeding from aggressive administration of fluid aimed at achieving hypervolemia. Isotonic crystalloid is the preferred agent for volume replacement. A 24-hour fluid balance is used with a goal of up to one liter positive balance to account for insensible losses, with urine output >0.5 cc/kg per hour and central venous pressure of 6–8 mm Hg as objective indicators of volume status. In patients with a persistent negative fluid balance, use of hydrocortisone (1,200 mg/day), promoting sodium retention in the kidneys, may be considered (48).
No well-controlled studies exist that answer whether blood pressure control in acute SAH influences rebleeding. It has been the consensus to maintain modest blood pressure elevation to systolic blood pressure <160 mmHg because it was shown to be not associated with re-bleeding. Blood pressure should be controlled with a titratable agent to balance the risk of rebleeding and maintenance of cerebral perfusion pressure (49).
In terms of blood glucose control, liberal glucose management (>220 mg/dL) is associated with increased infection risk and occurrence of vasospasm. On the other hand, in the NICE-SUGAR trial, SAH patients treated with insulin infusions to maintain tight glucose control (80–110 mg/dL) were found to have an increase in episodes of hypoglycemia and this was associated with more vasospasm and less favorable 3-month outcome (50). One study found improved outcomes in patients successfully treated to a target glucose range of 80–140 mg/dL (48).
Headaches are best initially treated with Acetaminophen 500–750 mg or Tramadol 25–50 mg given every 4 hours. If these are ineffective, then Fentanyl 12.5–25.0 mcg IV or Morphine Sulfate 2–4 mg IV every hour may be given (51). Measures to prevent deep venous thrombosis should be employed in all SAH patients with low molecular weight heparin or unfractionated heparin for prophylaxis but should be withheld in patients with unprotected aneurysms and expected to undergo surgery. These agents should be immediately started 24 hours after surgery until the patient is already ambulatory (48).
At present, no randomized, controlled trials are available to guide decisions on prophylaxis or treatment of seizures (52). However, short-term prophylactic antiepileptic therapy is still commonly used in patients with SAH based on the argument that seizures in acutely ill patients could lead to additional injury or rebleeding from an unsecured aneurysm (53). Also, the use of statins and magnesium sulphate were found to have no clinical benefit in SAH (54,55).
Prevention
Cigarette smoking is an independent and the most important risk factor for SAH with the adjusted relative risk of 3.0 for aneurysm rupture (56). It is possible that diet also increases the risk of SAH. In an epidemiological study of Finnish smokers who were monitored for 13 years, increased consumption of yogurt was associated with a higher risk of SAH while greater vegetable consumption is associated with a lower risk (57,58). Around 10% of SAH had a family history, hence, screening is recommended if two or more first degree relatives are affected (59).
Discussion
This literature review done on SAH highlights the numerous recommendations on its assessment, diagnosis and management from several studies and guidelines. Despite the numerous studies done on SAH, there are only a few high-level studies existing and most of them only tackle on a particular aspect of SAH. Most studies found were cohort studies and were based from expert opinion; hence, it might be helpful to have a clinical pathway suitable to the local situation.
In this clinical algorithm, patients presenting with acute severe headache that satisfies the modified Ottawa SAH rule should have a cranial CT scan done. If blood is present in the subarachnoid space, then an immediate CTA is warranted. If no SAH is seen but still highly suspicious of it, then lumbar puncture should be done after 12 hours post-ictus. The presence of xanthochromia or blood in the third tube >100 RBCs/HPF is highly suggestive of SAH and a CTA should also be done. If the plain cranial CT scan revealed diffuse SAH or the CTA turned out negative, the catheter angiography should be done. Patients confirmed to have SAH should be classified based on the Hunt and Hess scoring. Those with H&H grades 1–3 have good prognosis and aggressive surgical and medical management should be instituted. Those with H&H 4–5 should be managed medically before surgical management be contemplated. Surgical management consists of early surgical clipping or endovascular coiling to prevent re-bleeding, hematoma evacuation for hemorrhage greater than 30cc and EVD for acute hydrocephalus. Supportive therapy consisting of adequate oxygenation, maintenance of euvolemia, aggressive blood pressure control, glucose control, pain management, stress ulcer prophylaxis and venous thromboembolism prophylaxis should be observed. Prevention of delayed cerebral ischemia by administration of Nimodipine, aggressive thermoregulation of temperature less than 38.0°C and maintenance of Hgb >8 g/dL are recommended. Once Delayed cerebral ischemia sets in defined as the occurrence of focal neurologic impairment or decrease of at least two points in GCS, Triple-H therapy to increase the cerebral blood flow may be an option. In high grade SAH with neurologic improvement, surgery may be offered.
This literature review and development of clinical algorithm has its strengths and limitations that need to be acknowledged. It tries to deliberately encompass the complete process of assessment, diagnosis and management of acute SAH. All the articles included were reviewed for relevance and quality, however, it should be noted that no formal quality appraisal tool was used in the process. The selection and decision was based on the intellectual judgment of the authors. It should also be emphasized that the clinical algorithm was developed with consideration also of the current resources and funding available locally. Another limitation seen is that only English articles were included, hence the possibility that other relevant articles were excluded.
It is recommended that this clinical algorithm be tested for validity and reliability and be used in a longitudinal study to investigate its impact in the management of acute SAH.
Conclusions
Acute SAH remains to be a challenge to because of its high mortality and multiple complications. This literature review and clinical algorithm aims to guide Filipino health practitioners in the immediate recognition and appropriate institution of treatment for patients with SAH in order to reduce its morbidity and mortality.
Table S1
| Study | Study type | Level of evidence | Key results/Comments |
|---|---|---|---|
| Assessment | |||
| Bassi et al. | Retrospective study | Level III | Thunderclap headache was reliably present in 20.3% of patients |
| Linn et al. | Prospective study | Level III | Sudden severe headache within 1–5 minutes is present in ~50% of acute SAH |
| Edlow et al. | Expert Opinion | Level IV | Warning headache is an indication of unrecognized SAH warranting work-up |
| Fontanarosa | Expert Opinion | Level IV | The most common symptoms of SAH are headache, nausea, vomiting and loss of consciousness |
| de Falco | Systematic review | Level III | Sentinel headache is present in 10–43% of patients with SAH |
| Hauerberg et al. | Prospective study | Level III | Approximately 15% of patients with SAH were misdiagnosed. |
| Vermeulen et al. | Prospective study | Level III | 1 in 20 of SAH patients were misdiagnosed hence the need for heightened suspicion |
| Perry et al. | Cohort study | Level IIb | Of the 2,131 patients, the decision rule >40, neck stiffness, loss of consciousness or onset on exertion is 98.5% sensitive and 27.5% specific for SAH |
| Bonita | Case-control study | Level IV | Those who smoke and with hypertension were at increased risk for SAH |
| Diagnosis | |||
| van der Wee et al. | Prospective study | Level III | SAH may show normal CT findings hence lumbar puncture is done if SAH is suspected. |
| Kassell et al. | Prospective study | Level IIA | There is no difference in early and delayed surgery in SAH |
| Perry et al. | Prospective study | Level III | CT scan is 92.9% sensitive and 100% specific when done within 6 hours of headache. |
| Cortnum et al. | Retrospective study | Level III | CT scan is 100% sensitive after day 1–5 after SAH |
| Morgenstern et al. | Prospective study | Level III | CT scan is sufficient to exclude 97.5% of SAH in patients presenting with worst headache |
| O’Neill et al. | Retrospective study | Level III | A significant proportion of SAH patients are diagnosed with lumbar puncture |
| Vermeulen et al. | Retrospective study | Level III | Xantochromia and not blood-stained CSF are important in the diagnosis of SAH |
| Agid et al. | Retrospective study | Level III | 64-slice CTA is an accurate tool in detective acute SAH. |
| Kelliny et al. | Retrospective study | Level III | CTA is 96.4% sensitive and 96.0% specific in detection of aneurysmal rupture |
| Delgado et al. | Prospective study | Level III | Catheter angiography should be done seven days after SAH if with previous negative imaging |
| Management | |||
| Cha et al. | Retrospective study | Level III | Rebleeding occurs in the earlier period during SAH |
| Molyneux et al. | Randomized controlled trial | Level IB | Long-term outcome for SAH is better in endovascular coiling compared to neurosurgical clipping |
| Bracard et al. | Retrospective study | Level III | Early surgery in high-grade SAH is a feasible option |
| Starke et al. | Prospective study | Level IIB | Antifibrinolytic treatment was found to decrease risk of rebleeding but RCTs are still needed |
| de los Reyes et al. | Retrospective study | Level III | Coiling followed by ICH evacuation is associated with faster time to aneurysm protection |
| Gigante et al. | Expert opinion | Level IV | No definitive guideline for external ventricular drain is available |
| Rajshekhar et al. | Retrospective study | Level III | Routine ventriculostomy should be considered for all SAH patients with altered sensorium and acute hydrocephalus |
| Klopfenstein et al. | Randomized controlled trial | Level IB | Gradual weaning of ventricular drainage provided no advantage compared to rapid weaning in SAH |
| Kramer et al. | Retrospective study | Level III | Modified Fisher and Claassen scales are superior in predicting vasospasm and poor outcome |
| Dorhout et al. | Meta-analysis | Level IA | Nimodipine reduce the risk of poor outcome and secondary ischemia after aneurysmal SAH |
| Pickard et al. | Randomized controlled trial | Level IB | Oral Nimodipine reduces cerebral infarction and improves outcome after SAH |
| Oliveira-Filho et al. | Prospective study | Level III | Fever (T>38.3) is associated with vasospasm and poor outcome in SAH. |
| Dhar et al. | Prospective study | Level III | Blood transfusion in anemic patients with SAH resulted in a significant rise in cerebral oxygen delivery |
| Dankbaar et al. | Meta-analysis | Level IB | Triple-H therapy showed no positive effect in improving cerebral blood flow in SAH |
| Diringer et al. | Expert opinion | Level IV | – |
| Liu-Deryke et al. | Retrospective study | Level III | Nicardipine and Labetolol offer similar tolerability for blood pressure control for SAH |
| NICE-SUGAR Study Investigators | Randomized controlled trial | Level IB | Intensive blood sugar control increases risk for mortality in critically ill patients |
| Mahon et al. | Expert opinion | Level IV | – |
| Gilmore et al. | Expert opinion | Level IV | – |
| Deutschman et al. | Expert opinion | Level IV | – |
| Vergouwen et al. | Randomized controlled trial | Level IB | There is no beneficial effect of Simvastatin in SAH |
CTA, CT angiography; SAH, subarachnoid hemorrhage.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2017.02.11). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Feigin VL, Rinkel GJ, Lawes CM, et al. Risk factors for subarachnoid hemorrhage: an updated systematic review of epidemiological studies. Stroke 2005;36:2773-80. [Crossref] [PubMed]
- de Rooij NK, Linn FH, van der Plas JA, et al. Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatry 2007;78:1365-72. [Crossref] [PubMed]
- Connolly ES, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the Management of Aneurysmal Subarachnoid Hemorrhage: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2012;43:1711-37. [Crossref] [PubMed]
- Bassi P, Bandera R, Loiero M, et al. Warning signs in subarachnoid hemorrhage: a cooperative study. Acta Neurol Scand 1991;84:277-81. [Crossref] [PubMed]
- Linn FH, Rinkel GJ, Algra A, et al. Headache characteristics in subarachnoid haemorrhage and benign thunderclap headache. J Neurol Neurosurg Psychiatry 1998;65:791-3. [Crossref] [PubMed]
- Edlow JA, Caplan LR. Avoiding pitfalls in the diagnosis of subarachnoid hemorrhage. N Engl J Med 2000;342:29-36. [Crossref] [PubMed]
- Fontanarosa PB. Recognition of subarachnoid hemorrhage. Ann Emerg Med 1989;18:1199-205. [Crossref] [PubMed]
- de Falco FA. Sentinel headache. Neurol Sci 2004;25:S215-7. [Crossref] [PubMed]
- Hauerberg J, Andersen BB, Eskesen V, et al. Importance of the recognition of a warning leak as a sign of a ruptured intracranial aneurysm. Acta Neurol Scand 1991;83:61-4. [Crossref] [PubMed]
- Vermeulen MJ, Schull MJ. Missed diagnosis of subarachnoid hemorrhage in the emergency department. Stroke 2007;38:1216-21. [Crossref] [PubMed]
- Perry JJ, Stiell IG, Sivilotti ML, et al. Clinical decision rules to rule out subarachnoid hemorrhage for acute headache. JAMA 2013;310:1248-55. [Crossref] [PubMed]
- Bonita R. Cigarette smoking, hypertension and the risk of subarachnoid hemorrhage: a population-based case-control study. Stroke 1986;17:831-5. [Crossref] [PubMed]
- van der Wee N, Rinkel GJ, Hasan D, et al. Detection of subarachnoid haemorrhage on early CT: is lumbar puncture still needed after a negative scan? J Neurol Neurosurg Psychiatry 1995;58:357-9. [Crossref] [PubMed]
- Kassell NF, Torner JC, Haley EC Jr, et al. The International Cooperative Study on the Timing of Aneurysm Surgery. Part 1: Overall management results. J Neurosurg 1990;73:18-36. [Crossref] [PubMed]
- Perry JJ, Stiell IG, Sivilotti ML, et al. Sensitivity of computed tomography performed within six hours of onset of headache for diagnosis of subarachnoid haemorrhage: prospective cohort study. BMJ 2011;343:d4277. [Crossref] [PubMed]
- Vos PE, Zwienenberg M, O'Hannian KL, et al. Subarachnoid haemorrhage following rupture of an ophthalmic artery aneurysm presenting as traumatic brain injury. Clin Neurol Neurosurg 2000;102:29-32. [Crossref] [PubMed]
- Cortnum S, Sørensen P, Jørgensen J. Determining the sensitivity of computed tomography scanning in early detection of subarachnoid hemorrhage. Neurosurgery 2010;66:900-2; discussion 903. [PubMed]
- Morgenstern LB, Luna-Gonzales H, Huber JC Jr, et al. Worst headache and subarachnoid hemorrhage: prospective, modern computed tomography and spinal fluid analysis. Ann Emerg Med 1998;32:297-304. [PubMed]
- O'Neill J, McLaggan S, Gibson R. Acute headache and subarachnoid haemorrhage: a retrospective review of CT and lumbar puncture findings. Scott Med J 2005;50:151-3. [Crossref] [PubMed]
- Vermeulen M, Hasan D, Blijenberg BG, et al. Xanthochromia after subarachnoid haemorrhage needs no revisitation. J Neurol Neurosurg Psychiatry 1989;52:826-8. [Crossref] [PubMed]
- Agid R, Lee SK, Willinsky RA, et al. Acute subarachnoid hemorrhage: using 64-slice multidetector CT angiography to "triage" patients' treatment. Neuroradiology 2006;48:787-94. [Crossref] [PubMed]
- Kelliny M, Maeder P, Binaghi S, et al. Cerebral aneurysm exclusion by CT angiography based on subarachnoid hemorrhage pattern: a retrospective study. BMC Neurol 2011;11:8. [Crossref] [PubMed]
- Delgado Almandoz JE, Jagadeesan BD, et al. Diagnostic yield of repeat catheter angiography in patients with catheter and computed tomography angiography negative subarachnoid hemorrhage. Neurosurgery 2012;70:1135-42. [Crossref] [PubMed]
- Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg 1968;28:14-20. [Crossref] [PubMed]
- Seder DB, Mayer SA. Critical care management of subarachnoid hemorrhage and ischemic stroke. Clin Chest Med 2009;30:103-22. viii-ix. [Crossref] [PubMed]
- Takagi K, Tamura A, Nakagomi T, et al. How should a subarachnoid hemorrhage grading scale be determined? A combinatorial approach based solely on the Glasgow Coma Scale. J Neurosurg 1999;90:680-7. [Crossref] [PubMed]
- Cha KC, Kim JH, Kang HI, et al. Aneurysmal rebleeding: factors associated with clinical outcome in the rebleeding patients. J Korean Neurosurg Soc 2010;47:119-23. [Crossref] [PubMed]
- Molyneux AJ, Kerr RS, Yu LM, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005;366:809-17. [Crossref] [PubMed]
- Bracard S, Lebedinsky A, Anxionnat R, et al. Endovascular treatment of Hunt and Hess grade IV and V aneuryms. AJNR Am J Neuroradiol 2002;23:953-7. [PubMed]
- Starke RM, Kim GH, Fernandez A, et al. Impact of a protocol for acute antifibrinolytic therapy on aneurysm rebleeding after subarachnoid hemorrhage. Stroke 2008;39:2617-21. [Crossref] [PubMed]
- de los Reyes K, Patel A, Bederson JB, et al. Management of subarachnoid hemorrhage with intracerebral hematoma: clipping and clot evacuation versus coil embolization followed by clot evacuation. J Neurointerv Surg 2013;5:99-103. [Crossref] [PubMed]
- Rinne J, Hernesniemi J, Niskanen M, et al. Analysis of 561 patients with 690 middle cerebral artery aneurysms: anatomic and clinical features as correlated to management outcome. Neurosurgery 1996;38:2-11. [Crossref] [PubMed]
- Hasan D, Tanghe HL. Distribution of cisternal blood in patients with acute hydrocephalus after subarachnoid hemorrhage. Ann Neurol 1992;31:374-8. [Crossref] [PubMed]
- Gigante P, Hwang BY, Appelboom G, et al. External ventricular drainage following aneurysmal subarachnoid haemorrhage. Br J Neurosurg 2010;24:625-32. [Crossref] [PubMed]
- Rajshekhar V, Harbaugh RE. Results of routine ventriculostomy with external ventricular drainage for acute hydrocephalus following subarachnoid haemorrhage. Acta Neurochir (Wien) 1992;115:8-14. [Crossref] [PubMed]
- Klopfenstein JD, Kim LJ, Feiz-Erfan I, et al. Comparison of rapid and gradual weaning from external ventricular drainage in patients with aneurysmal subarachnoid hemorrhage: a prospective randomized trial. J Neurosurg 2004;100:225-9. [Crossref] [PubMed]
- Weir B. Subarachnoid Hemorrhage: Causes and Cures. New York: Oxford University Press, 1998.
- Vergouwen MD, Vermeulen M, van Gijn J, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke 2010;41:2391-5. [Crossref] [PubMed]
- Kramer AH, Hehir M, Nathan B, et al. A comparison of 3 radiographic scales for the prediction of delayed ischemia and prognosis following subarachnoid hemorrhage. J Neurosurg 2008;109:199-207. [Crossref] [PubMed]
- Dorhout Mees SM, Rinkel GJ, Feigin VL, et al. Calcium antagonists for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev 2007;CD000277. [PubMed]
- Pickard JD, Murray GD, Illingworth R, et al. Effect of oral nimodipine on cerebral infarction and outcome after subarachnoid haemorrhage: British aneurysm nimodipine trial. BMJ 1989;298:636-42. [Crossref] [PubMed]
- Oliveira-Filho J, Ezzeddine MA, Segal AZ, et al. Fever in subarachnoid hemorrhage: relationship to vasospasm and outcome. Neurology 2001;56:1299-304. [Crossref] [PubMed]
- Dorhout Mees SM, Luitse MJ, van den Bergh WM, et al. Fever after aneurysmal subarachnoid hemorrhage: relation with extent of hydrocephalus and amount of extravasated blood. Stroke 2008;39:2141-3. [Crossref] [PubMed]
- Dhar R, Zazulia AR, Videen TO, et al. Red blood cell transfusion increases cerebral oxygen delivery in anemic patients with subarachnoid hemorrhage. Stroke 2009;40:3039-44. [Crossref] [PubMed]
- Gathier CS, van den Bergh WM, Slooter AJ, et al. HIMALAIA (Hypertension Induction in the Management of AneurysmaL subArachnoid haemorrhage with secondary IschaemiA): a randomized single-blind controlled trial of induced hypertension vs. no induced hypertension in the treatment of delayed cerebral ischemia after subarachnoid hemorrhage. Int J Stroke 2014;9:375-80. [Crossref] [PubMed]
- Wilson SR, Hirsch NP, Appleby I. Management of subarachnoid haemorrhage in a non-neurosurgical centre. Anaesthesia 2005;60:470-85. [Crossref] [PubMed]
- Dankbaar JW, Slooter AJ, Rinkel GJ, et al. Effect of different components of triple-H therapy on cerebral perfusion in patients with aneurysmal subarachnoid haemorrhage: a systematic review. Crit Care 2010;14:R23. [Crossref] [PubMed]
- Diringer MN, Bleck TP, Claude Hemphill J 3rd, et al. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical Care Society's Multidisciplinary Consensus Conference. Neurocrit Care 2011;15:211-40. [Crossref] [PubMed]
- Liu-Deryke X, Janisse J, Coplin WM, et al. A comparison of nicardipine and labetalol for acute hypertension management following stroke. Neurocrit Care 2008;9:167-76. [Crossref] [PubMed]
- NICE-SUGAR Study Investigators. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009;360:1283-97. [Crossref] [PubMed]
- Mahon P, Smith B, Browne J, et al. Effective headache management in the aneurysmal subarachnoid patient: A literature review. Br J of Neurosci Nurs 2012;8:89-93. [Crossref]
- Gilmore E, Choi HA, Hirsch LJ, et al. Seizures and CNS hemorrhage: spontaneous intracerebral and aneurysmal subarachnoid hemorrhage. Neurologist 2010;16:165-75. [Crossref] [PubMed]
- Deutschman CS, Haines SJ. Anticonvulsant prophylaxis in neurological surgery. Neurosurgery 1985;17:510-7. [Crossref] [PubMed]
- Vergouwen MD, Meijers JC, Geskus RB, et al. Biologic effects of simvastatin in patients with aneurysmal subarachnoid hemorrhage: a double-blind, placebo-controlled randomized trial. J Cereb Blood Flow Metab 2009;29:1444-53. [Crossref] [PubMed]
- Wong GK, Poon WS, Chan MT, et al. Intravenous magnesium sulphate for aneurysmal subarachnoid hemorrhage (IMASH): a randomized, double-blinded, placebo-controlled, multicenter phase III trial. Stroke 2010;41:921-6. [Crossref] [PubMed]
- Juvela S. Risk factors for multiple intracranial aneurysms. Stroke 2000;31:392-7. [Crossref] [PubMed]
- Larsson SC, Männistö S, Virtanen MJ, et al. Dairy foods and risk of stroke. Epidemiology 2009;20:355-60. [Crossref] [PubMed]
- Larsson SC, Männistö S, Virtanen MJ, et al. Dietary fiber and fiber-rich food intake in relation to risk of stroke in male smokers. Eur J Clin Nutr 2009;63:1016-24. [Crossref] [PubMed]
- Sundquist J, Li X, Sundquist K, Hemminki K. Risks of subarachnoid hemorrhage in siblings: a nationwide epidemiological study from Sweden. Neuroepidemiology 2007;29:178-84. [Crossref] [PubMed]
Cite this article as: De Roxas RC, Barcelon EA, Dioquino-Maligaso CP. Developing an evidence-based clinical algorithm for the assessment, diagnosis and management of acute subarachnoid hemorrhage: a review of literature. J Xiangya Med 2017;2:24.