Lung volume reduction surgery in selected patients with severe emphysema: significant benefit with low peri-operative risk
Introduction
The National Emphysema Treatment Trial (NETT) was published in 2003 and was the first randomized multi-center trial comparing lung volume reduction surgery (LVRS) with medical treatment in patients with severe emphysema (1). With more than 1,200 patients participating, significant improvements of lung function, walking distance, quality of life and even survival was shown for the LVRS group. Also, a number of large case series reported this treatment success (2-5). Previously, the NETT reported about a high risk group of included patients who showed high mortality after LVRS (6). This report was published in 2001 and described 69 patients with very low values of forced expiratory volume in 1 second (FEV1 below 20% predicted) either with homogeneous emphysema or with a very low carbon monoxide diffusion capacity (DLCO below 20% predicted). These patients had a postoperative 30-day-mortality up to 25% and during the further on-going trial this combination of characteristics was an exclusion criterion. Nevertheless, the publication was titled “Patients at high risk of death after lung volume reduction surgery” and therefore was interpreted mistakably. The emerging LVRS received a setback even before the promising results of the completed NETT were published two years later. This misunderstanding is still an issue and LVRS is perceived as heavily complicated (7). Many physicians remain unaware of the benefits of LVRS (8). This review emphasizes the technique of LVRS, its patient selection criteria and the results. Nowadays very low perioperative morbidity and mortality rates are achieved and this beneficial outcome may encourage thoracic surgeons and chest physicians to consider their emphysema patients as potential candidates for LVRS.
Rationale
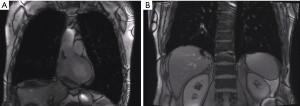
LVRS downsizes the hyperinflated lung to a more physiologic size and the diaphragmatic dome moves upwards (9). Figure 1 shows a MRI of a patient before and after bilateral LVRS. Maximal ventilator and exercise capacity are improved (10). Static lung volumes as functional residual capacity and residual volume (RV) are reduced while lung elastic recoil is increased. Therefore the airflow obstruction and hyperinflation are decreased (11). Both heterogeneous and homogeneous emphysema are responsible for the hyperinflation and therefore patients with both types of morphology can profit from LVRS (12).
Selection criteria
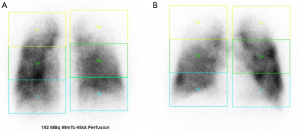
Patient selection is a key issue and should be performed at high volume centers with an interdisciplinary emphysema board (13). Computer tomography should be performed in every patient with chronic obstructive pulmonary disease (COPD). With evidence of emphysema on CT scan (Figure 2) and lung function criteria as listed in Table 1 the patient should be referred and discussed at the emphysema board. Best evidence exists for patients with heterogeneous, upper-lobe predominant emphysema (1). Patients with lower-lobe predominant disease seem to profit as well in a short-term after LVRS (5). The key issue in patient selection must be the hyperinflation: high total lung capacity (TLC) and RV values and a RV/TLC-ratio above 60. Ventilation-perfusion scans are helpful in identifying the most destroyed parts of the lung (Figure 3) (6).
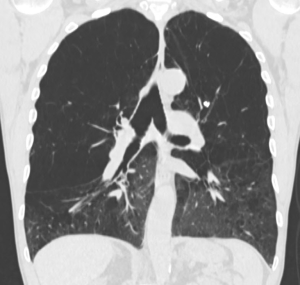
Table 1
| Variables | Inclusion | Exclusion |
|---|---|---|
| Patient | Nicotine abstention >4 months; passed pulmonary rehabilitation | Daily steroid intake >20 mg |
| CT morphology | Lung emphysema | Significant bronchiectasis |
| Lung function | FEV1 <45%; TLC >100%; RV >150%; RV/TLC >60% | FEV1 <20% and diffusion capacity <20% |
| 6MWD (m) | <450 | <140 |
| Gas exchange | paCO2 >6.7 Pa; paO2 <6.0 Pa |
LVRS, lung volume reduction surgery; CT, computer tomography; FEV1, forced expiratory volume in 1 second; TLC, total lung capacity; RV, residual volume; RV/TLC, hyperinflation; 6MWD, 6 minute walking distance.
Flattened diaphragms on coronal CT scans and X-rays help to identify the severity of hyperinflation. Patients often tell by themselves they feel bloated. Also they report about the inability to eat more than just small portions. A rigorous cardiac assessment is necessary. Echocardiography should be performed in every candidate. Patients with an ejection fracture below 30%, a history of myocardial infarction within the last 6 months and suspicion of pulmonary hypertension (>35 mmHg) should be excluded or sent for further investigation by a cardiologist (1,7,8).
Nevertheless, even significant benefits have been reported about LVRS in patients with homogeneous emphysema (14,15). This group of patients might be included after gaining large experience in patients with heterogeneous morphology.
Technique
The golden standard is the video-assisted thoracoscopic surgery (VATS) LVRS, already described in 1996 (16). Most studies describe a three-portal approach (4,5). Our group always had good results with one-staged bilateral LVRS in patients with bilateral disease but other groups might prefer bi-staged procedures (4,17).
For one-staged bilateral LVRS the patient lies in a supine position with both arms raised. Both sides can be approached without changing the patient’s position. General anesthesia is performed with a double lumen tube and additional epidural anesthesia.
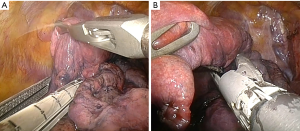
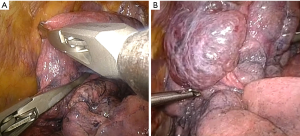
The defined target area is prepared with compressing the lung parenchyma along the line of the proposed staple line with a clamp or grasp (Figure 4). The intraoperative macroscopic appearance of the target area must correspondence to the CT scan. Manipulation and contact with the lung should be limited as the emphysematous lung is very thin and even touching it with the stapler can make a hole. Before introducing the stapler, the lung should be aligned so it can slide easily across the lung. Resection with a stapler along the pre-compressed region of the lung is performed (Figure 5). We generally use a 60 mm endostapler with 4.8 mm staples. In upper-lobe predominant emphysema the resection often starts at the level of the vena azygos or the aortic arch, respectively. For resection of the dorsal part the stapler is tilted or the lung pulled upwards to keep the dome-like shape of the lung. The pulmonary ligament is released if there is a significant air space in the apical part. One chest tube is placed. When the air leak is zero or small we proceed to the contralateral side.
After operation the double lumen tube is changed to a laryngeal mask. Patients are then extubated in the operation room. The chest tubes are connected to a water seal with suction of −5 cm H2O.
Results
The postoperative 90-day mortality in the final NETT was 5.2% (1). This mortality rate was achieved in 511 non-high-risk patients with upper-lobe predominant emphysema.
In 2003, Ciccone and colleagues reported a 90-day mortality of 4% in 250 patients with heterogeneous emphysema (5). Ginsberg and colleagues even reported a zero 6-month mortality rate in 91 consecutive patients with upper-lobe predominant emphysema (18). In 2014, Rathinam and colleagues published a 30-day mortality rate of 3% in 265 VATS patients (19). Additional evidence was made for patients with severe hyperinflation and airflow obstruction to benefit from LVRS even if their emphysema was non-heterogeneously distributed. 30-day mortality rates between 2.4% (15) and 9.3% (20) were reported.
Pulmonary complications concern mainly postoperative airleaks. We define a prolonged airleak as chest tube duration longer than 7 days. In our own experience this has to be expected in about at least 30% of all patients after LVRS. Ginsburg et al. report a rate of 57% (18) whereas in the NETT (information available for 522 patients) median air leak duration was 7 days and in 12% of patients it lasted at least 30 days (21). The 4.4% of all patients underwent re-operation due to air leak.
The mainly published median postoperative hospitalization time is between 8 and 14 days (5,18,19,22).
In the NETT, a substantial survival advantage was found after LVRS compared with medical therapy (8). Lung function improves significantly after LVRS. The beneficial effect remains but declines during 5 years (3,5,13). 94% of the 250 patients with heterogeneous emphysema from Ciccone et al. had a significant postoperative improvement (5). The mean increase of FEV1 was 54% after six months. After 5 years, the mean change in FEV1 was still an improvement of 7%, and 53% of patients still had an increase relative to the preoperative value. The 6 minute walking distance (6MWD) improve by 46% after 6 months and was still increased by 25% after 5 years. The Zurich group showed a mean change in FEV1 of 41% after 6 months including patients with heterogeneous and homogeneous emphysema (4). Brenner and colleagues (2) showed an mean improvement of 69% after 6 months in their study with 269 patients with heterogeneous emphysema.
LVRS and transplantation
Lung transplantation (LTx) also offers significant improvement of quality of life in selected patients with emphysema compared to medical therapy (23). Usually, LVRS is offered at a less progressed stage of disease. Nevertheless, a highly selected group of patients might profit from both procedures (24). Facing the problems with organ shortage, LVRS can be a bridge to transplantation. The experience from Zurich shows no survival disadvantage for LTx patients with previous LVRS (4).
Perspective
Nowadays endoscopic lung volume reduction (ELVR) procedures are emerging and also show promising results.
Endobronchial valve treatment in heterogeneous emphysema shows improvement of 24.8% in FEV1% predicted after 3 months (25) and of 17% in homogeneous emphysema (26). Pneumothorax rates between 8% and 25.6% were reported. During the first 3 months, 2 deaths occurred in the BeLieFeR-HIFi-Study (25 patients with valve treatment) and no deaths in the IMPACT-study (43 patients with valve treatment). The VENT trial randomized 220 patients to endobronchial valve treatment and 110 patients to standard medical care. There was a 6-months mortality rate of 2.8% after endobronchial valve treatment (6 patients of 220) (27). Coils are another well researched endobronchial treatment procedure. One recent multicenter randomized study shows a median improvement of its primary endpoint at 12 months, the 6MWD of 10.3 meters (28). The recent published REVOLENS-trial, another multicenter randomized trial, showed an at least 54 m gain in 36% of patients treated with endobronchial coils compared with medical-care-only patients (29). Improvement of FEV1% predicted differs between 3.8% and 9% in these two trials. Major complications (i.e., pneumonia, pneumothorax, hemoptysis, COPD exacerbations) occurred in 34.8% and 52%, respectively.
Regarding ELVR results, LVRS still remains the gold standard for lung volume reduction. Bronchoscopic procedures, especially valves show impressive results but also have to deal with major complications.
The initially discussed concerns about the high mortality and morbidity of LVRS are not justified regarding the literature. With its promising improvements of lung function and its advantages for survival LVRS should be considered in every emphysema patient matching the proper selection criteria.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2017.05.04). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fishman A, Martinez F, Naunheim K, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348:2059-73. [Crossref] [PubMed]
- Brenner M, McKenna RJ JR, Chen JC, et al. Relationship between amount of lung resected and outcome after lung volume reduction surgery. Ann Thorac Surg 2000;69:388-93. [Crossref] [PubMed]
- Flaherty KR, Kazerooni EA, Curtis JL, et al. Short-term and long-term outcomes after bilateral lung volume reduction surgery: prediction by quantitative CT. Chest 2001;119:1337-46. [Crossref] [PubMed]
- Tutic M, Lardinois D, Imfeld S, et al. Lung-volume reduction surgery as an alternative or bridging procedure to lung transplantation. Ann Thorac Surg 2006;82:208-13. [Crossref] [PubMed]
- Ciccone AM, Meyers BF, Guthrie TJ, et al. Long-term outcome of bilateral lung volume reduction in 250 consecutive patients with emphysema. J Thorac Cardiovasc Surg 2003;125:513-25. [Crossref] [PubMed]
- Chandra D, Lipson DA, Hoffman EA, et al. Perfusion scintigraphy and pa- tient selection for lung volume reduction surgery. Am J Respir Crit Care Med 2010;182:937-46. [Crossref] [PubMed]
- National Emphysema Treatment Trial Research Group. Patients at high risk of death after lung-volume-reduction surgery. N Engl J Med 2001;345:1075-83. [Crossref] [PubMed]
- Criner GJ, Cordova F, Sternberg AL, et al. The National Emphysema Treat- ment Trial (NETT) Part II: Lessons learned about lung volume reduction surgery. Am J Respir Crit Care Med 2011;184:881-93. [Crossref] [PubMed]
- McNulty W, Jordan S, Hopkinson NS. Attitudes and access to lung volume reduction surgery for COPD: a survey by the British Thoracic Society. BMJ Open Respir Res 2014;1:e000023. [Crossref] [PubMed]
- Cassart M, Hamacher J, Verbandt Y, et al. Effects of lung volume reduction surgery for emphysema on diaphragm dimensions and configuration. Am J Respir Crit Care Med 2001;163:1171-5. [Crossref] [PubMed]
- Fessler HE, Permutt S. Lung volume reduction surgery and airflow limitation. Am J Respir Crit Care Med 1998;157:715-22. [Crossref] [PubMed]
- Sciurba FC, Rogers FM, Keenan RJ, et al. Improvement in pulmonary function and elastic recoil after lung-reduction surgery for diffuse emphysema. N Engl J Med 1996;334:1095-9. [Crossref] [PubMed]
- Bloch KE, Georgescu CL, Russi EW, et al. Gain and subsequent loss of lung function after lung volume reduction surgery in cases of severe emphysema with different morphologic patterns. J Thorac Cardiovasc Surg 2002;123:845-54. [Crossref] [PubMed]
- Weder W, Tutic M, Bloch KE. Lung volume reduction surgery in non-heterogeneous emphysema. Thorac Surg Clin 2009;19:193-9. [Crossref] [PubMed]
- Weder W, Tutic M, Lardinois D, et al. Persistent benefit from lung volume reduction surgery in patients with homogeneous emphysema. Ann Thorac Surg 2009;87:229-36. [Crossref] [PubMed]
- Bingisser R, Zollinger A, Hauser M, et al. Bilateral volume reduction surgery for diffuse pulmonary emphysema by video-assisted thoracoscopy. J Thorac Cardiovasc Surg 1996;112:875-82. [Crossref] [PubMed]
- Oey IF, Morgan MD, Spyt TJ, et al. Staged bilateral lung volume reduction surgery - the benefits of a patient-led strategy. Eur J Cardiothorac Surg 2010;37:846-52. [Crossref] [PubMed]
- Ginsburg ME, Thomashow BM, Bulman WA, et al. The safety, efficacy, and durability of lung-volume reduction surgery: A 10-year experience. J Thorac Cardiovasc Surg 2016;151:717-24.e1. [Crossref] [PubMed]
- Rathinam S, Oey I, Steiner M, et al. The role of the emphysema multidisciplinary team in a successful lung volume reduction surgery programme. Eur J Cardiothorac Surg 2014;46:1021-6. [Crossref] [PubMed]
- Wisser W, Tschernko E, Wanke T, et al. Functional improvements in ventilatory mechanics after lung volume reduction surgery for homogeneous emphysema. Eur J Cardiothorac Surg 1997;12:525-30. [Crossref] [PubMed]
- DeCamp MM, Blackstone EH, Naunheim KS, et al. Patient and surgical factors influencing air leaks after lung volume reduction surgery: lessons learned from the National Emphysema Treatment Trial. Ann Thorac Surg 2006;82:197-206; discussion 206-7. [Crossref] [PubMed]
- Pompeo E, Marino M, Nofroni I, et al. Reduction pneumoplasty versus respiratory rehabilitation in severe emphysema: a randomized study. Pulmonary Emphysema Research Group. Ann Thorac Surg 2000;70:948-53. [Crossref] [PubMed]
- Backhus L, Sargent J, Cheng A, et al. Outcomes in lung transplantation after previous lung volume reduction surgery in a contemporary cohort. J Thorac Cardiovasc Surg 2014;147:1678-83.e1. [Crossref] [PubMed]
- Kostron A, Horn-Tutic M, Franzen D, et al. Repeated lung volume reduction surgery is successful in selected patients. Eur J Cardiothorac Surg 2015;48:710-5. [Crossref] [PubMed]
- Davey C, Zoumot Z, Jordan S, et al. Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR-HIFi study): a randomised controlled trial. Lancet 2015;386:1066-73. [Crossref] [PubMed]
- Valipour A, Slebos DJ, Herth F, et al. Endobronchial Valve Therapy in Patients with Homogeneous Emphysema. Results from the IMPACT Study. Am J Respir Crit Care Med 2016;194:1073-82. [Crossref] [PubMed]
- Sciurba FC, Ernst A, Herth FJ, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med 2010;363:1233-44. [Crossref] [PubMed]
- Sciurba FC, Criner GJ, Strange C, et al. Effect of Endobronchial Coils vs Usual Care on Exercise Tolerance in Patients With Severe Emphysema: The RENEW Randomized Clinical Trial. JAMA 2016;315:2178-89. [Crossref] [PubMed]
- Deslée G, Mal H, Dutau H. Lung Volume Reduction Coil Treatment vs Usual Care in Patients With Severe Emphysema: The REVOLENS Randomized Clinical Trial. JAMA 2016;315:175-84. [Crossref] [PubMed]
Cite this article as: Caviezel C. Lung volume reduction surgery in selected patients with severe emphysema: significant benefit with low peri-operative risk. J Xiangya Med 2017;2:48.