
Totally laparoscopic intragastric surgery for gastric submucosal tumors located near the esophagogastric junction
Introduction
Gastric submucosal tumors (SMTs) are a relatively rare type of tumors and account for less than two percent of gastric tumors. Surgical resection is the main treatment, however no standard surgical approach could be refer due to the feature of particular entity and demanding technique. With the progress of laparoscopic instrument and the improvement of surgical technology, the less invasive laparoscopic or endoscopic method, accounting to the different growth pattern, had been widely used in the management of gastric SMTs and been considered as standard approach (1). However, for SMTs located in special parts such as the lesser curvature or posterior, the original laparoscopic or endoscopic approach is difficult to perform, and may lead to complications such as cardiac stricture or fistula, although rendezvous maneuver or transgastric approach had been reported, and various advances in the devices and techniques used for endoscopic ESD, proximal gastrectomy or total gastrectomy were reported with impaired quality of life. Intragastric resection was first introduced by Ohashi (2) in 1995, and this procedure had evolved to a more maturely procedure, and make the local resection of gastric SMTs in special parts possible without disturbing or impairing the cardia function. Herein, we design the total laparoscopic intragastric surgery (T-LIGS), this procedure was conduct without the use of gastroscope, the ‘pneumogastrium’ was established by rigid laparoscope, and the tumors were resected by conventional laparoscopic instruments. We applied this procedure to 14 cases, and the clinicopathological results were analyzed as described underneath.
Methods
General information
T-LIGS was conducted in 14 consecutive patients including 4 males and 10 females, the diagnosis was made by gastroscopy and the distance from the cardia and the caliber of the SMTs were measured. All cases of this novel procedure were conducted in the Department of Gastrointestinal Nutrition and Hernia Surgery, the Second Hospital of Jilin University. Extra-preoperative workup including endoscopic ultrasound (EUS) and enhanced computer tomography (CT) of the stomach, is all in a bid to confirm the feature of SMT including the location diameter and the circumferential setting, also to estimate the lymph node metastasis or involvement of adjacent organs. Data were retrieved preoperatively, including information on patient demographics, preoperative workup, operative findings, postoperative course, morbidity, and mortality, pathologic findings and follow-up. Informed consent and ethic committee approval was obtained before the initiation of this review. The clinicopathological feature was showed in Table 1.
Table 1
| Parameter | n |
|---|---|
| No. of patients | 14 |
| Age (years) | 55.8±8.8 |
| Sex | |
| Male | 4 |
| Female | 10 |
| Body mass index (kg/m2) | 21.0±3.0 |
| Chief complaint | |
| Epigastric discomfort | 4 |
| Epigastralgia | 5 |
| Positive fecal occult blood | 2 |
| Physical examination | 3 |
| Co-morbidity | 5/9 |
| Diabetes | 1 |
| Coronary artery disease | 1 |
| Cirrhosis | 1 |
| Hypertension | 2 |
| Previous operation history | |
| Appendicectomy (open/laparoscopic) | 1/1 |
| Cholecystectomy | 1 |
| Oophorocystectomy | 1 |
| Cesarean section | 1 |
| Tumor size (mm) | 16.4±5.3 [15–25] |
| Distance from the EGJ (mm) | 15.3±7.9 [0–30] |
| Tumor location | |
| Posterior wall | 5 |
| Anterior wall | 3 |
| Lesser curvature | 3 |
| Greater curvature | 0 |
| Fundus | 2 |
| EGJ | 1 |
| Preoperative CT confirmation (yes/no) | 9/5 |
| Preoperative gastroscopy positioning (yes/no) | 4/10 |
TLIGS, totally laparoscopic intragastric surgery; SMT, submucosal tumor; EGJ, esophagogastric junction; CT, computer tomography.
A protocol for the application of this novel procedure was approved by the Ethics Committee of the Second Hospital of Jilin University and written informed consent was obtained from each patient.
Surgical methods
The laparoscopy was performed under general anesthesia with intravenous antibiotic prophylaxis. The patient was in modified Trendelenburg position with legs separated, and the surgeon was positioned on the left with the camera operator between the legs and the first assistant on the right side. The protocol of the T-LIGS procedure were summarized as follows (Figure 1): (I) a umbilical 10 mm trocar with self-fixing device (fix-aid trocar) was inserted into the abdominal cavity as the observation hole and a 30° laparoscope was introduced, pneumoperitoneum was instituted and set at 12 mmHg. Two 5 mm trocar (conventional) was insert in the right upper quadrant to assist in the exposure of the surgical field and subsequent gastric puncture, two additional 5 and 12 mm fix-aid trocar was inserted in the left upper quadrant with a distance of 3 cm or larger, and expected as the main and auxiliary operation hole for introgastric surgery. (II) Abdominal adhesions around and stomach was separated if exist, and stomach mobilization with exploration of the gastric SMTs showed by preoperative CT or gastroscope positioning was achieved, the growth pattern was also probed. Jejunum blockage was achieved by using laparoscopic intestinal blocking to clamp the proximal jejunum about 10–20 cm distal from the ligament of Treitz. (III) Suitable puncture point was chosen in the anterior wall of the stomach, and 2 traction suture was used before full-thickness incision of the gastric wall by electric hook with length about 0.5 cm; the observation hole was switch to the conventional in the right abdomen, and the insertion of the first intraluminal trocar was conducted under the surveillance of laparoscopy; “pneumogastrium” was instituted through this trocar and set at 12 mmHg, and the two additional 5 and 12 mm fix-aid trocar was switched into the gastric cavity at a reasonable distance with the assistance procedure from the conventional trocar. (IV) The location of gastric SMT and its growth pattern was confirmed, the tumor size and the distance from the esophagogastric junction (EGJ) was calibrated under intraluminal laparoscopy. The proposed resection line was marked by electrocautery circumferentially and the maximum resected specimen size (mm) was measured to confirm adequate margins, the non-expose semi-thickness dissection of gastric SMT was initiated from the proximal circumferential with adequate surgical margins by monopolar electrocautery, caution and meticulous manipulation was paid to avoid bleeding, perforation, or rupture. (V) A retrieval bag was inserted into the gastric lumen through and the specimen was placed into it, the surgical site of the mucosal defect was examination to exclude bleeding or perforation before continuous suture was used to close the wound. Intraoperative frozen section pathological examination was proceeding, and the gastric puncture point was closed by sutured.
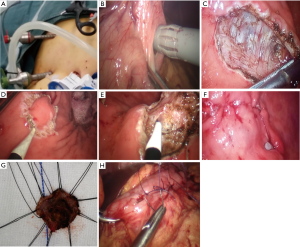
For all the patients, the operating time (min), blood loss (mL), tumor growth pattern, intraoperative complications, maximum resected specimen diameter (mm), minimum surgical margin (mm) were measured, and the postoperative complication, pathological diagnosis, the time of resumption to oral intake (days) and postoperative hospital stay (days) were measured postoperatively.
Results
All the 14 patients underwent successful T-LIGS without open conversion or operative failure. The operation time was 71.1±22.2 min and the blood loss was 9.3±7.0 mL. All patients received complete resection with a negative margin. No operation-related mortality or severe morbidity was observed in our series. One patient experienced postoperative gastroparesis, another patient experienced surgical site infection, both patient recovered after conservative treatment. The mean postoperative length of hospital stay was 6.6±1.7 days. In pathology there were nine cases of gastrointestinal stromal tumor (GIST), two cases of neurofibroma, two cases of neuroendocrine tumor (NET), and one case of mucosa associated lymphoid tissue lymphoma. In follow-up, one patient experienced of stenosis of the cardia, no tumor recurrence was confirmed during a mean follow-up of 13.9±7.4 months. The result of T-LIGS was list on Table 2.
Table 2
| Parameter | Outcomes |
|---|---|
| Operating time (min) | 71.1±22.2 [45–110] |
| Blood loss (mL) | 9.3±7.0 [5-30] |
| Maximum resected specimen size (mm) | 27.5±7.0 [20-40] |
| Minimum surgical margin (mm) | 6.4±2.3 [5-10] |
| Intra-operative complication | |
| Bleeding | 0 |
| Perforation | 0 |
| Resort to gastroscope | 1 |
| Open conversion | 1 |
| Tumor growth pattern | |
| Endogastric | 12 |
| Exogastric | 0 |
| Transgastric | 2 |
| Pathological diagnosis | |
| GIST | 9 |
| Neurofibroma | 2 |
| NET | 2 |
| MALT-L | 1 |
| Postoperative complication | |
| Fistula | 0 |
| Bleeding | 0 |
| Surgical site infection | 1 |
| Gastroparesis | 1 |
| Resumption of oral intake (days) | 3.3±1.2 [2-5] |
| Postoperative hospital stay (days) | 6.6±1.7 [5-11] |
| Follow-up | |
| Visit/loss | 11/3 |
| Follow-up period (months) | 6.0±2.3 [3-9] |
| Long-term complications | |
| Recurrence | 0 |
| Cardiac stricture | 1 |
T-LIGS, totally laparoscopic intragastric surgery; SMT, submucosal tumor; GIST, gastrointestinal stromal tumor; NET, neuroendocrine tumor; MALT-L, mucosa associated lymphoid tissue lymphoma.
Discussion
As a relatively rare tumor gastric SMTs is often diagnosed incidentally by routine gastroscopy (3), the preoperative pathological diagnosis were often difficult to confirm (4), in order to insure adequate margin surgeons often removed moderate surrounding tissues, once the diagnosis of gastric SMTs was made, although lymphadenectomy was not generally required. The gastric SMTs can be classified into exogenous, endogenous and transgastric growth based on its growth pattern, with the endogenous was much more difficult to cope with. The pathology of gastric SMTs can be divided into two categories, respectively the mesenchymal tumors and non-mesenchymal tumors, and GIST was the most common type of mesenchymal tumors. Endoscopic resection had becoming an important means of treatment of gastric SMTs, however, when a tumor is too large or located in the posterior wall or gastric fundus, gastroscopy is very difficult to achieve, even using special equipment by experienced doctors, it still carry a certain degree of intraoperative or delayed complications such as perforation and abscess, which limited its application in special parts of the gastric SMTs (5). The ESD treatment of SMT has a high morbidity such as massive bleeding and perforation of stomach, and not suitable for resection of muscle. With the progress of laparoscopic technology and the improvement of equipment, a variety of ways of laparoscopic treatment of gastric SMTs had been derived from the operation of the traditional laparoscopic partial gastrectomy (LAP), and either transgastric or intragastric resection can be selected accounting to the position and growth pattern of gastric SMTs (6,7). The selection of surgical approach for gastric SMTs was basically on the operating surgeon’s discretion. For gastric SMTs located near the EGJ, the posterior, or the lesser curvature, especially in the endogenous growth pattern, where the operative field is limited and the expose of the tumor is difficult, the use of traditional method is restricted, proximal gastrectomy or total gastrectomy may need to avoid the complications such as cardiac stricture or fistula (8). Ohashi (2) first reported the intraluminal use of laparoscopic electrocauterization for the treatment of early cancer in 1995, and named it as laparoscopic intragastric surgery (LIGS). And various methods of intragastric wedge resection have been reported depend on the characteristics of the tumor. However, all these methods depend on endoscope, either for the inflated of airflow via oral endoscopy, or for provide an endoscopic view of surveillance for intragastric resection, and the specimens were retrieved through the oral route (9). However, in particular situations even when the stomach was inflated, the application of an endo-linear stapler is not easy, because of the relatively large of the tumor comparing to the restriction of the length of the stapler in a narrow operation field (10). We use the rigid laparoscope to substitute the endoscope to establish the “pneumogastrium”, and all the procedure was carried out within the conventional laparoscopic instrument under, name it as the T-LIGS.
The results and the clinical effect of T-LIGS technology was evaluated and showed in the table. In our application experience, T-LIGS had some drawbacks: firstly, it does not provide “full-thickness” but “semi-full” thickness resection of the stomach. Secondly, the possible spillage of gastric content during intragastric access or procedure may cause intra-abdominal infection or surgical site infection. Thirdly, it is hard to handle and open conversion may be needed, when incidentally exposed the pseudocapsule of gastric SMTs. These deficiencies are common problems of LIGS, technically, T-LIGS can comply with all the oncologic principles of surgical management of gastric SMTs, and there is no obvious inferiority of T-LIGS as compare to traditional LIGS. We only experienced one case of surgical site infection in the umbilical trocar site which symptomatic remit after drainage and dressing change. As far as open conversion be concerned, although we do not encounter, we regard that oncological principle be prior to minimally invasive, and we insist that T-LIGS is not an technique-damned procedure, and laparoscopic surgeons can master it easily after learning curve. Compared with other procedure T-LIGS has some of its own characteristics (11): firstly, the operation time T-LIGS is shorten as a result of compromising the intraoperative endoscopy without increasing the trauma; it is applicable to the gastric SMTs located <1 cm from the EGJ, which is the forbidden zone for conventional LAP, and it is suitable for SMTs with diameter >2 cm and superior to the existing endoscopic ESD technology. Secondly, the establishment of pneumoperitoneum as the initial step T-LIGS not only facilitate the gastric puncture, but also provide favorable conditions for exploration of the gastric SMTs and associate lymph node, which is suitable for patients with abdominal operation history; It can meet the special requirements of lymph node dissection, and simultaneously procedure can be conducted for concomitant disease such as obesity (12,13). Thirdly, the indication of T-LIGS can be expanded to the surgical management of foreign body, bezoar, and incarcerated gastric band (14); and it is suitable for proposed full-thickness excision, after phased exogastric seromuscular layer resection and endogastric submucosal dissection and wall-inversion maneuver, without causing deformity or perforation and provide excellent oncological outcome (15).
In conclusion, T-LIGS is an safe and feasible procedure that may coincidence with the oncological demand and may expanded indications of laparoscopic surgery for gastric SMTs (16), however as a small sample study, the results of this study should be carefully interpreted, before strong evidences be provided by more well-design and large-scale studies.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2017.04.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Second Hospital of Jilin University and written informed consent was obtained from each patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lee HH, Hur H, Jung H, et al. Analysis of 151 consecutive gastric submucosal tumors according to tumor location. J Surg Oncol 2011;104:72-5. [Crossref] [PubMed]
- Ohashi S. Laparoscopic intraluminal (intragastric) surgery for early gastric cancer. A new concept in laparoscopic surgery. Surg Endosc 1995;9:169-71. [Crossref] [PubMed]
- Ryu KJ, Jung SR, Choi JS, et al. Laparoscopic resection of small gastric submucosal tumors. Surg Endosc 2011;25:271-7. [Crossref] [PubMed]
- Nishida T, Kawai N, Yamaguchi S, et al. Submucosal tumors: comprehensive guide for the diagnosis and therapy of gastrointestinal submucosal tumors. Dig Endosc 2013;25:479-89. [Crossref] [PubMed]
- Kang SH, Lee K, Lee HW, et al. Delayed Perforation Occurring after Endoscopic Submucosal Dissection for Early Gastric Cancer. Clin Endosc 2015;48:251-5. [Crossref] [PubMed]
- Lee HH, Hur H, Jung H, et al. Laparoscopic wedge resection for gastric submucosal tumors: a size-location matched case-control study. J Am Coll Surg 2011;212:195-9. [Crossref] [PubMed]
- Xu X, Chen K, Zhou W, et al. Laparoscopic transgastric resection of gastric submucosal tumors located near the esophagogastric junction. J Gastrointest Surg 2013;17:1570-5. [Crossref] [PubMed]
- Hwang SH, Park DJ, Kim YH, et al. Laparoscopic surgery for submucosal tumors located at the esophagogastric junction and the prepylorus. Surg Endosc 2009;23:1980-7. [Crossref] [PubMed]
- Tagaya N, Mikami H, Kubota K. Laparoscopic resection of gastrointestinal mesenchymal tumors located in the upper stomach. Surg Endosc 2004;18:1469-74. [Crossref] [PubMed]
- Shim JH, Lee HH, Yoo HM, et al. Intragastric approach for submucosal tumors located near the Z-line: a hybrid laparoscopic and endoscopic technique. J Surg Oncol 2011;104:312-5. [Crossref] [PubMed]
- Boulanger-Gobeil C, Gagné JP, Julien F, et al. Laparoscopic Intragastric Resection: An Alternative Technique for Minimally Invasive Treatment of Gastric Submucosal Tumors. Ann Surg 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Hashimoto K, Seki Y, Kasama K. Laparoscopic intragastric surgery and laparoscopic roux-y gastric bypass were performed simultaneously on a morbidly obese patient with a gastric submucosal tumor: a report of a case and review. Obes Surg 2015;25:564-7. [Crossref] [PubMed]
- Kim DJ, Kim W. A case of single lymph node metastasis near the common hepatic artery following a curative endoscopic resection for gastric mucosal cancer. Gastric Cancer 2014;17:387-91. [Crossref] [PubMed]
- Rodarte-Shade M, Barrera GT, Arredondo JF, et al. Hybrid technique for removal of eroded adjustable gastric band. JSLS 2013;17:338-41. [Crossref] [PubMed]
- Goto O, Takeuchi H, Kawakubo H, et al. Feasibility of non-exposed endoscopic wall-inversion surgery with sentinel node basin dissection as a new surgical method for early gastric cancer: a porcine survival study. Gastric Cancer 2015;18:440-5. [Crossref] [PubMed]
- Mino JS, Guerron AD, Monteiro R, et al. Long-term outcomes of combined endoscopic/laparoscopic intragastric enucleation of presumed gastric stromal tumors. Surg Endosc 2016;30:1747-53. [Crossref] [PubMed]
Cite this article as: Ma Z, Liu T, Fang X, Liu J, Sun P, Zhu J. Totally laparoscopic intragastric surgery for gastric submucosal tumors located near the esophagogastric junction. J Xiangya Med 2017;2:52.


