Does serum bilirubin prevent cardiovascular disease?
Introduction
Oxidative stress has been implicated in the pathogenic mechanisms of most non-communicable diseases, including metabolic syndrome (MetS) (1), atherosclerosis and cancer. LDL cholesterol is rendered more atherogenic by oxidative modification (2) and many carcinogens create free oxygen radicals that damage DNA and other cellular structures, initiating and promoting tumor development (3). Therefore, antioxidant agents have been extensively evaluated in the prevention of cardiovascular disease (CVDs) and cancer. Vitamin E has been shown to reduce atherosclerotic lesions in animals (4), smooth muscle cell proliferation (5), platelet adherence and aggregation (6). Epidemiological data indicate a negative association between cardiovascular or cancer risk and vitamin E intake from dietary sources and/or supplements (7). However, most randomized controlled trials have failed to confirm a role for vitamin E supplementation in cardiovascular prevention (8-12). Indeed, vitamin E has been reported to have no significant effect on myocardial infarction, stroke, cardiovascular death, unstable angina, revascularization, total mortality (13) or diabetes (14,15). The findings from trials of cancer chemoprevention have also been disappointing (16-18). This situation is called the antioxidant paradox. Bilirubin has been recognized as a potent antioxidant, so does serum bilirubin prevent metabolic or atherosclerotic CVD?
Bilirubin as an antioxidant
The catabolism of heme by heme oxygenase generates carbon monoxide, free iron, and biliverdin, which is rapidly converted to bilirubin by ubiquitously expressed biliverdin reductase. Bilirubin efficiently scavenges a wide range of physiological oxidants by electron donation. In this process, it is often reconverted to biliverdin, but biliverdin reductase quickly regenerates bilirubin, thereby greatly boosting its antioxidant potential. Bilirubin suppresses the oxidation of lipids in liposomes more than vitamin E, which is regarded as the best antioxidant of lipid peroxidation (19,20). The water-soluble glutathione primarily protects water soluble proteins, whereas the lipophilic bilirubin protects lipids from oxidation (21). Serum bilirubin has been demonstrated to be a major contributor to the total antioxidant capacity in blood plasma (22) and is proven to have anti-inflammatory (23) and anti-platelet (24) properties. However, serum bilirubin is not an ideal marker of oxidative stress because serum levels of bilirubin are influenced by various factors related to hemoglobin metabolism as well as conjugation and excretion of bilirubin apart from oxidative stress.
Bilirubin as an anti-hypercholesterolemic agent
Apart from its antioxidant and anti-inflammatory properties, bilirubin is suggested to have a hypocholesterolemic property (25-27). The profound hypocholesterolemia and hypotriglyceridemia found in a murine model of hyperbilirubinemia (Gunn rat) suggests that an increase in serum bilirubin levels may decrease serum levels of cholesterol and triglycerides (25,26). Gilbert’s Syndrome is associated with a mutation in the hepatic Uridine Glucuronosyl Transferase 1A1 (UGT1A1) gene promoter, reducing UGT1A1 activity, which normally conjugates bilirubin, allowing its elimination from the blood. Individuals with Gilbert’s syndrome demonstrate mildly elevated plasma antioxidant capacity due to the elevated levels of unconjugated bilirubin. In a case-control study, when subdivided into younger and older cohorts, older patients with Gilbert’s syndrome demonstrated reduced levels of total cholesterol and LDL cholesterol compared with controls (26). The author observed that serum bilirubin was significantly negatively associated with incident hyper-LDL cholesterolemia in a health screening population (27). The exact mechanisms underlying this negative association between baseline serum bilirubin and incident hyper-LDL cholesterolemia are unknown. However, it has been suggested that increased bilirubin levels affect lipid homeostasis through increased intestinal cholesterol secretion, reduced hepatic cholesterol synthesis and increased biliary cholesterol excretion (28). Recently, a direct effect of bilirubin on cholesterol efflux was demonstrated and is associated with decreased ABCA1 protein expression (29).
Bilirubin as an anti-adiposity agent
Bilirubin has a new function as a ligand for PPARα. Stec et al. demonstrated that bilirubin can bind directly to PPARα and increase transcriptional activity (30). When they compared the PPARα transcriptional activation of biliverdin with that of a known PPARα ligand, fenofibrate, fenofibrate and biliverdin have similar activation properties. Treatment of 3T3-L1 adipocytes with biliverdin suppressed lipid accumulation and upregulated PPARα target genes. They treated wild-type and PPARα KO mice on a high fat diet with fenofibrate or bilirubin and found that both signal through PPARα dependent mechanisms. Furthermore, the effect of bilirubin on lowering glucose and reducing body fat percentage was blunted in PPARα KO mice (30). These data suggest a new function for bilirubin as an agonist of PPARα, which mediates the protection from adiposity. Involvement of AMPK pathway was also suggested for the protection of Gilbert’s syndrome from adiposity (31).
Cross-sectional negative associations between serum bilirubin and metabolic and atherosclerotic CVD as well as CVD risk factors
Serum bilirubin has been shown to be negatively associated with MetS in Chinese children, adolescents and adults (32,33): Korean men and women (34,35) and nonsmoking Japanese men and women (36) in cross-sectional studies. Serum bilirubin levels are negatively associated with impaired flow-mediated vasodilation and carotid intima-media thickness in men and women (37). Patients with Gilbert’s syndrome had low levels of oxidative stress associated with enhancement of endothelium-dependent vasodilation (38). A low serum bilirubin concentration is associated with coronary artery calcification (39). Serum bilirubin has been demonstrated to be negatively associated with coronary heart disease (40-42), stroke (42,43) and peripheral artery disease (44) in cross-sectional studies. One author reported that serum bilirubin is negatively associated with hemoglobin A1c, independently of other cardiovascular risk factors, in healthy men and women in a cross-sectional study suggesting an negative inverse association between bilirubin and diabetes (45). Fukui et al. observed a significant cross-sectional negative association between serum bilirubin and albuminuria in patients with diabetes (46). The presence of silent cerebral infarction increases the risk of transient ischemia attack, symptomatic stroke, CVD and dementia. A cross-sectional study demonstrated that a higher serum bilirubin was associated with a lower risk of silent cerebral infarction (47). These cross-sectional studies are summarized in Table 1.
Table 1
| Studies (reference) | Subjects | Risk factors or diseases | Relationships |
|---|---|---|---|
| Lin et al. (32) | 4,723 children and adolescents | Metabolic syndrome | Negative association |
| Wu et al. (33) | 1,423 adults | Metabolic syndrome | Negative association |
| Choi et al. (34) | 12,342 adults | Metabolic syndrome | Negative association |
| Kwon et al. (35) | 5,266 women | Metabolic syndrome | Negative association |
| Oda et al. (48) | 3,681 adults | Metabolic syndrome | Negative association |
| Erdogan et al. (37) | 91 middle-aged subjects | Endothelial dysfunction | Negative association |
| Carotid intima-media thickness | Negative association | ||
| Maruhashi et al. (38) | 108 men with Gilbert’s syndrome vs. controls | Endothelial dysfunction | Negative association |
| Oxidative stress markers | Negative association | ||
| Tanaka et al. (39) | 637 patients | Coronary artery calcification | Negative association |
| Schwertner et al. (40) | 877 men | Coronary heart disease | Negative association |
| Hopkins et al. (41) | 161 subjects with EFCHD and 155 controls | Coronary heart disease | Negative association |
| Oda et al. (42) | 3,375 men and 2,069 women | Coronary heart disease | Negative association |
| Stroke | Negative association | ||
| Perlstein et al. (43) | 13,214 adults | Stroke | Negative association |
| Perlstein et al. (44) | 7,075 adults | Peripheral artery disease | Negative association |
| Oda et al. (45) | 2,500 men and 1,680 women | Hemoglobin a1c | Negative association |
| Fukui et al. (46) | 633 diabetics | Albuminuria | Negative association |
| Pulse wave velocity | Negative association | ||
| Li et al. (47) | 1,831 men and 1,034 women | Silent cerebral infarction | Negative association |
| Pulse wave velocity | Negative association |
EFCHD, early familial coronary heart disease.
Conflicting results in longitudinal studies on the association between serum bilirubin and metabolic and atherosclerotic CVD as well as CVD risk factors
A U-shaped relationship was observed between serum bilirubin and risk of coronary heart disease in middle-aged British men in a large, long-term prospective study (49). Another nested case-control study also observed a U-shaped relationship between bilirubin concentration and coronary heart disease risk in middle-aged men (50). Serum bilirubin levels are determined both genetically and environmentally. Linkage studies have identified a major locus at the chromosome 2q telomere that affects bilirubin concentrations and a candidate gene in the linkage region encodes UGT1A1 (51). The insertion of a TA in the TATAA box of the gene promotor region, an allele designated UGT1A1*28, decreases gene transcription. Individuals homozygous for UGT1A1*28 (genotype 7/7) have increased serum bilirubin levels compared with carriers of the 6 allele (52). In the Rotterdam Study, homozygote or heterozygote UGT1A1*28 allele carriers were not associated with myocardial infarction (53). However, homozygote UGT1A1*28 allele carriers with elevated serum bilirubin concentrations exhibited a strong negative association with a reduced risk of CVD in the Framingham heart study offspring cohort (54). McArdle et al. reported that the UGT1A1*28 genotype was not significantly associated with any of the traditional CVD risk factors, and they suggested that the CVD benefits associated with increased serum bilirubin may in part be mediated by the early regulation of vascular structure and reactivity (55). A genome-wide association meta-analysis for total serum bilirubin levels showed a strong association between the UGT1A1 rs6742078 genotype and serum bilirubin (56). To test the hypothesis that elevated serum bilirubin is causally related to decreased risk of CVD, Stender et al. genotyped rs6742078 in the UGT1A1 gene in 67,068 individuals, 11,686 of whom had coronary heart disease (57). UGT1A1 rs6742078 TT versus GG genotype was not significantly associated with CVD risk; the TT versus GG genotype was associated with odds ratios (ORs) [95% confidence intervals (CIs)] of 1.03 (0.96–1.11) (P=0.73) for coronary heart disease and 1.01 (0.92–1.12) (P=0.68) for myocardial infarction (57). In their meta-analysis of 14,711 cases and 60,324 controls, the random effects OR (95% CI) of coronary heart disease for genotypes with approximately 100% increased bilirubin levels versus reference genotypes was 1.01 (0.88–1.16), and they concluded that serum bilirubin is not causally associated with the risk of coronary heart disease (57).
A study from Korea reported that the lowest serum bilirubin level category (bilirubin ≤0.32 mg/dL) was an independent risk factor for coronary heart disease (58). Another study from Germany observed that a 1-SD higher bilirubin level was associated with a 19% lower frequency of incident cardiovascular events in an age- and gender-adjusted Cox regression analysis [hazard ratio 95% CI: 0.81 (0.68; 0.96), P=0.014]; however, this hazard ratio became insignificant after adjustment for traditional cardiovascular risk factors [0.87 (0.73; 1.04), P=0.13] (59). A study suggested that serum bilirubin might have some protective function against ischemic stroke risk in Korean men, excluding subjects with Gilbert’s syndrome (60). We suggested that serum bilirubin might be a negative predictor of end-stage kidney disease in Japanese using hospital-based data (61). Sakoh et al. reported that a lower serum bilirubin concentration was independently associated with adverse renal outcomes in Japanese patients with moderate-to-severe chronic kidney disease (62). Riphagen et al. observed a protective effect of bilirubin against progression of diabetic nephropathy in patients with type 2 diabetes in a post hoc analysis using Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) trial and Irbesartan Diabetic Nephropathy Trial (IDNT) data (63). Okada et al. suggested that low serum bilirubin levels could be a risk factor for the development of albuminuria in Japanese patients with type 2 diabetes (64). Mashitani et al. reported that serum bilirubin levels were associated with the progression of diabetic nephropathy in Japanese type 2 diabetic patients, independent of possible confounders and suggested that serum bilirubin levels might be the link in the correlation between hemoglobin levels and nephropathy progression (65).
A longitudinal study reported that serum bilirubin levels were negatively associated with the incidence of MetS in healthy Korean men (66). Among individual components of MetS, bilirubin was significantly associated with only incident hypertriglyceridemia (66). Another longitudinal study reported that decreased serum bilirubin levels predicted MetS in healthy middle-aged non-smoking Taiwanese men (67). It is reported that genotype-phenotype relationships regarding obesity traits were influenced by smoking status (68,69). Effects of genotypes linked to TB levels on incidence of MetS, diabetes and CVDs may be different between smokers and non-smokers. However, the author found that serum bilirubin levels could not predict the development of MetS in a Japanese population (48). Lee et al. also reported that no association was observed between baseline TB and incident MetS in men, but a significant inverse association was observed in women, which became insignificant after adjusted for insulin resistance (70).
A retrospective longitudinal study reported that serum bilirubin levels predicted diabetes in healthy Korean men (71). However, the authors of that study did not include baseline fasting glucose in the adjusted covariates of their multivariable-adjusted models (71). The author observed that incident prediabetes was not significantly associated with the quintiles of serum bilirubin but was positively associated with a 1 SD increase in serum bilirubin levels in non-smoking men, although serum bilirubin levels were significantly negatively associated with prevalent prediabetes in non-smokers (36). Therefore, a negative association between serum bilirubin levels and incident prediabetes seemed to be unlikely (36).
These conflicting results in longitudinal studies on the association between serum bilirubin and metabolic and atherosclerotic diseases are summarized in Table 2.
Table 2
| Studies (reference) | Subjects | Follow-up periods | Risk factors | Outcomes | Results |
|---|---|---|---|---|---|
| Breimer et al. (49) | 7,685 men | 11.5 years | Serum bilirubin | Coronary heart disease | U-shaped association |
| Troughton et al. (50) | 10,593 men | 5 years | Serum bilirubin | Coronary heart disease | U-shaped association |
| Bosma et al. (53) | 185 cases vs. 370 controls | Case-control study | UGT1A1*28 | Myocardial infarction | No significant association |
| Lin et al. (54) | 1,780 individuals | Mendelian randomization | UGT1A1*28 | Coronary heart disease | Negative association |
| McArdle et al. (55) | 868 individuals | Mendelian randomization | UGT1A1*28 | CVD risk factors | No significant association |
| Stender et al. (57) | 67,068 individuals | Mendelian randomization | UGT1A1 rs6742078 | Coronary heart disease | No significant association |
| Song et al. (58) | 8,593 individuals | 4 years | Serum bilirubin | Coronary heart disease | Negative association |
| Mahabadi et al. (59) | 3,553 individuals | 9.1 years | Serum bilirubin | Cardiovascular events | No significant association |
| Kimm et al. (60) | 41,054 men† | 13 years | Serum bilirubin | Ischemic stroke | Negative association |
| 37,670 women† | 13 years | Serum bilirubin | Ischemic stroke | No significant association | |
| Oda et al. (61) | 6,251 patients | 1 year | Serum bilirubin | ESKD | Negative association |
| Sakoh et al. (62) | 279 CKD patients | 21 months | Serum bilirubin | Progression of CKD | Negative association |
| Riphagen et al. (63) | 1,498 diabetics in RENAAL | 3.4 years | Serum bilirubin | Progression of DN | Negative association |
| 1,707 diabetics in IDNT | 2.6 years | Serum bilirubin | Progression of DN | Negative association | |
| Okada et al. (64) | 320 diabetics | 3.2 years | Serum bilirubin | Proteinuria | Negative association |
| Mashitani et al. (65) | 2,511 diabetics | 1.4 years | Serum bilirubin | Macroalbuminuria | Negative association |
| Adjusted for hemoglobin | No significant association | ||||
| Lee et al. (66) | 6,205 men | 4 years | Serum bilirubin | Metabolic syndrome | Negative association |
| Huang et al. (67) | 377 nonsmoking men | 7.6 years | Serum bilirubin | Metabolic syndrome | Negative association |
| Oda et al. (48) | 2,558 men and women | 4 years | Serum bilirubin | Metabolic syndrome | No significant association |
| Lee et al. (70) | 6,890 men | 5 years | Serum bilirubin | Metabolic syndrome | No significant association |
| 4,723 women | 5 years | Serum bilirubin | Metabolic syndrome | Negative association | |
| Adjusted for insulin resistance | No significant association | ||||
| Jung et al. (71) | 5,960 men | 4 years | Serum bilirubin | Diabetes | Negative association |
| Oda (36) | 2,149 individuals | 6 years | Serum bilirubin | Prediabetes | No significant association |
†, excluding Gilbert’s syndrome. UGT1A1, uridine diphosphate glucuronosyltransferase1A1; CVD, cardiovascular disease; ESKD, end-stage kidney disease; CKD, chronic kidney disease; RENAAL, Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan Study; IDNT, Irbesartan Diabetic Nephropathy Trial; DN, diabetic nephropathy.
Considerable fluctuation of bilirubin levels
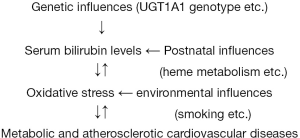
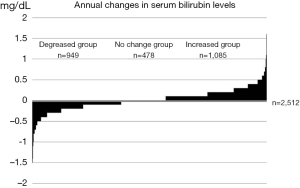
Serum bilirubin levels are determined both genetically and environmentally and fluctuate throughout one’s life, as illustrated in Figure 1. The annual changes in the serum total bilirubin levels of 2,512 apparently healthy subjects who visited our medical check-up center are presented in Figure 2. Considerable changes were observed in serum bilirubin levels over the course of one year.
Conclusions
The above-mentioned cross-sectional and longitudinal studies suggest that postnatal metabolic and atherosclerotic diseases sometimes cause oxidative stress and reduce serum bilirubin levels, and antioxidant agents such as bilirubin do not always prevent metabolic and atherosclerotic diseases, although certain genetically hyperbilirubinemic individuals may have lower CVD risk than the general population. A similar situation has been documented for antioxidant vitamins and is called the antioxidant paradox. Further prospective studies are required to confirm whether serum bilirubin prevents the development of non-communicable disease such as MetS, diabetes and CVD.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2017.07.05). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Oda E. Metabolic syndrome: its history, mechanisms, and limitations. Acta Diabetol 2012;49:89-95. [Crossref] [PubMed]
- Steinberg D, Parthasarathy S, Carew TE, et al. Beyond cholesterol: modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med 1989;320:915-24. [Crossref] [PubMed]
- Prasad KN, Edwards-Prasad J. Vitamin E and cancer prevention: recent advances and future potentials. J Am Coll Nutr 1992;11:487-500. [Crossref] [PubMed]
- Verlangieri AJ, Bush MJ. Effects of d-α-tocopherol supplementation on experimentally induced primate atherosclerosis. J Am Coll Nutr 1992;11:131-138. [PubMed]
- Meydani M, Vitamin E. Lancet 1995;345:170-5. [Crossref] [PubMed]
- Steiner M. Influence of vitamin E on platelet function in humans. J Am Coll Nutr 1991;10:466-73. [Crossref] [PubMed]
- Jha P, Flather M, Lonn E, et al. The antioxidant vitamins and cardiovascular disease: a critical review of epidemiologic and clinical trial data. Ann Intern Med 1995;123:860-72. [Crossref] [PubMed]
- Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet 1999;354:447-55. [Crossref] [PubMed]
- Heart Outcomes Prevention Evaluation Study Investigators. Vitamin E supplementation and cardiovascular events in high-risk patients. N Engl J Med 2000;342:154-60. [Crossref] [PubMed]
- de Gaetano GCollaborative Group of the Primary Prevention Project. Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Collaborative Group of the Primary Prevention Project. Lancet 2001;357:89-95. [Crossref] [PubMed]
- Heart Protection Collaborative Group. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20 536 high-risk individuals: a randomized placebo-controlled trial. Lancet 2002;360:23-33. [Crossref] [PubMed]
- Vivekananthan DP, Penn MS, Sapp SK, et al. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet 2003;361:2017-2023. [Crossref] [PubMed]
- Curtis AJ, Bullen M, Piccenna L, et al. Vitamin E supplementation and mortality in healthy people: a meta-analysis of randomised controlled trials. Cardiovasc Drugs Ther 2014;28:563-73. [Crossref] [PubMed]
- Song Y, Cook NR, Albert CM, et al. Effects of vitamins C and E and beta-carotene on the risk of type 2 diabetes in women at high risk of cardiovascular disease: a randomized controlled trial. Am J Clin Nutr 2009;90:429-37. [Crossref] [PubMed]
- Kataja-Tuomola MK, Kontto JP, Männistö S, et al. Intake of antioxidants and risk of type 2 diabetes in a cohort of male smokers. Eur J Clin Nutr 2011;65:590-7. [Crossref] [PubMed]
- Lonn E, Bosch J, Yusuf S, et al. Effects of long-term vitamin E supplementation on cardiovascular events and cancer:a randomized controlled trial. JAMA 2005;293:1338-47. [Crossref] [PubMed]
- The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med 1994;330:1029-35. [Crossref] [PubMed]
- Wang L, Sesso HD, Glynn RJ, et al. Vitamin E and C supplementation and risk of cancer in men: posttrial follow-up in the Physicians' Health Study II randomized trial. Am J Clin Nutr 2014;100:915-23. [Crossref] [PubMed]
- Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science 1987;235:1043-6. [Crossref] [PubMed]
- Wu TW, Fung KP, Yang CC. Unconjugated bilirubin inhibits the oxidation of human low-density lipoprotein better than Trolox. Life Sci 1994;54:477-81. [Crossref] [PubMed]
- Sedlak TW, Saleh M, Higginson DS, et al. Bilirubin and glutathione have complementary antioxidant and cytoprotective roles. Proc Natl Acad Sci USA 2009;106:5171-6. [Crossref] [PubMed]
- Frei B, Stocker R, Ames BN. Antioxidant defenses and lipid peroxidation in human blood plasma. Proc Natl Acad Sci USA 1988;85:9748-52. [Crossref] [PubMed]
- Vítek L, Schwertner HA. The heme catabolic pathway and its protective effects on oxidative stress-mediated diseases. Adv Clin Chem 2007;43:1-57. [Crossref] [PubMed]
- Kundur AR, Singh I, Bulmer AC. Bilirubin, platelet activation and heart disease: a missing link to cardiovascular protection in Gilbert's syndrome? Atherosclerosis 2015;239:73-84. [Crossref] [PubMed]
- Boon AC, Hawkins CL, Bisht K, et al. Reduced circulating oxidized LDL is associated with hypocholesterolemia and enhanced thiol status in Gilbert syndrome. Free Radic Biol Med 2012;52:2120-7. [Crossref] [PubMed]
- Wallner M, Marculescu R, Doberer D, et al. Protection from age-related increase in lipid biomarkers and inflammation contributes to cardiovascular protection in Gilbert's syndrome. Clin Sci (Lond) 2013;125:257-64. [Crossref] [PubMed]
- Oda E. A decrease in total bilirubin predicted hyper-LDL cholesterolemia in a health screening population. Atherosclerosis 2014;235:334-8. [Crossref] [PubMed]
- Bulmer AC, Verkade HJ, Wagner KH. Bilirubin and beyond: a review of lipid status in Gilbert's syndrome and its relevance to cardiovascular disease protection. Prog Lipid Res 2013;52:193-205. [Crossref] [PubMed]
- Wang D, Tosevska A, Heiß EH, et al. Bilirubin Decreases Macrophage Cholesterol Efflux and ATP-Binding Cassette Transporter A1 Protein Expression. J Am Heart Assoc 2017;6: [Crossref] [PubMed]
- Stec DE, John K, Trabbic CJ, et al. Bilirubin Binding to PPARα Inhibits Lipid Accumulation. PLoS One 2016;11:e0153427. [Crossref] [PubMed]
- Mölzer C, Wallner M, Kern C, et al. Features of an altered AMPK metabolic pathway in Gilbert's Syndrome, and its role in metabolic health. Sci Rep 2016;6:30051. [Crossref] [PubMed]
- Lin LY, Kuo HK, Hwang JJ, et al. Serum bilirubin is inversely associated with insulin resistance and metabolic syndrome among children and adolescents. Atherosclerosis 2009;203:563-8. [Crossref] [PubMed]
- Wu Y, Li M, Xu M, et al. Low serum total bilirubin concentrations are associated with increased prevalence of metabolic syndrome in Chinese. J Diabetes 2011;3:217-24. [Crossref] [PubMed]
- Choi SH, Yun KE, Choi HJ. Relationships between serum total bilirubin levels and metabolic syndrome in Korean adults. Nutr Metab Cardiovasc Dis 2013;23:31-7. [Crossref] [PubMed]
- Kwon KM, Kam JH, Kim MY, et al. Inverse association between total bilirubin and metabolic syndrome in rural Korean women. J Womens Health (Larchmt) 2011;20:963-9. [Crossref] [PubMed]
- Oda E. Cross-sectional and longitudinal associations between serum bilirubin and prediabetes in a health screening population. Can J Diabetes 2016;40:270-5. [Crossref] [PubMed]
- Erdogan D, Gullu H, Yildirim E, et al. Low serum bilirubin levels are independently and inversely related to impaired flow-mediated vasodilation and increased carotid intima-media thickness in both men and women. Atherosclerosis 2006;184:431-7. [Crossref] [PubMed]
- Maruhashi T, Soga J, Fujimura N, et al. Hyperbilirubinemia, augmentation of endothelial function, and decrease in oxidative stress in gilbert syndrome. Circulation 2012;126:598-603. [Crossref] [PubMed]
- Tanaka M, Fukui M, Tomiyasu K, et al. Low serum bilirubin concentration is associated with coronary artery calcification (CAC). Atherosclerosis 2009;206:287-91. [Crossref] [PubMed]
- Schwertner HA, Jackson WG, Tolan G. Association of low serum concentration of bilirubin with increased risk of coronary artery disease. Clin Chem 1994;40:18-23. [PubMed]
- Hopkins PN, Wu LL, Hunt SC, et al. Higher serum bilirubin is associated with decreased risk for early familial coronary artery disease. Arterioscler Thromb Vasc Biol 1996;16:250-5. [Crossref] [PubMed]
- Oda E, Kawai R. A possible cross-sectional association of serum total bilirubin with coronary heart disease and stroke in a Japanese health screening population. Heart Vessels 2012;27:29-36. [Crossref] [PubMed]
- Perlstein TS, Pande RL, Creager MA, et al. Serum total bilirubin level, prevalent stroke, and stroke outcomes: NHANES 1999-2004. Am J Med 2008;121:781-788.e1. [Crossref] [PubMed]
- Perlstein TS, Pande RL, Beckman JA, et al. Serum total bilirubin level and prevalent lower-extremity peripheral arterial disease: National Health and Nutrition Examination Survey (NHANES) 1999 to 2004. Arterioscler Thromb Vasc Biol 2008;28:166-72. [Crossref] [PubMed]
- Oda E, Kawai R. Bilirubin is negatively associated with hemoglobin A1c independently of other cardiovascular risk factors in apparently healthy Japanese men and women. Circ J 2011;75:190-5. [Crossref] [PubMed]
- Fukui M, Tanaka M, Shiraishi E, et al. Relationship between serum bilirubin and albuminuria in patients with type 2 diabetes. Kidney Int 2008;74:1197-201. [Crossref] [PubMed]
- Li RY, Cao ZG, Zhang JR, et al. Decreased serum bilirubin is associated with silent cerebral infarction. Arterioscler Thromb Vasc Biol 2014;34:946-51. [Crossref] [PubMed]
- Oda E, Aizawa Y. Total bilirubin is inversely associated with metabolic syndrome but not a risk factor for metabolic syndrome in Japanese men and women. Acta Diabetol 2013;50:417-22. [Crossref] [PubMed]
- Breimer LH, Wannamethee G, Ebrahim S, et al. Serum bilirubin and risk of ischemic heart disease in middle-aged British men. Clin Chem 1995;41:1504-8. [PubMed]
- Troughton JA, Woodside JV, Young IS, et al. Bilirubin and coronary heart disease risk in the Prospective Epidemiological Study of Myocardial Infarction (PRIME). Eur J Cardiovasc Prev Rehabil 2007;14:79-84. [Crossref] [PubMed]
- Lin JP, Cupples LA, Wilson PW, et al. Evidence for a gene influencing serum bilirubin on chromosome 2q telomere: a genomewide scan in the Framingham study. Am J Hum Genet 2003;72:1029-34. [Crossref] [PubMed]
- Lin JP, Schwaiger JP, Cupples LA, et al. Conditional linkage and genome-wide association studies identify UGT1A1 as a major gene for anti-atherogenic serum bilirubin levels – The Framingham Heart Study. Atherosclerosis 2009;206:228-33. [Crossref] [PubMed]
- Bosma PJ, van der Meer IM, Bakker CT, et al. UGT1A1*28 allele and coronary heart disease:the Rotterdam Study. Clin Chem 2003;49:1180-1. [Crossref] [PubMed]
- Lin JP, O'Donnell CJ, Schwaiger JP, et al. Association between the UGT1A1*28 allele, bilirubin levels, and coronary heart disease in the Framingham Heart Study. Circulation 2006;114:1476-81. [Crossref] [PubMed]
- McArdle PF, Whitcomb BW, Tanner K, et al. Association between bilirubin and cardiovascular disease risk factors: using Mendelian randomization to assess causal inference. BMC Cardiovasc Disord 2012;12:16. [Crossref] [PubMed]
- Johnson AD, Kavousi M, Smith AV, et al. Genome-wide association meta-analysis for total serum bilirubin levels. Hum Mol Genet 2009;18:2700-10. [Crossref] [PubMed]
- Stender S, Frikke-Schmidt R, Nordestgaard BG, et al. Genetically elevated bilirubin and risk of ischaemic heart disease: three Mendelian randomization studies and a meta-analysis. J Intern Med 2013;273:59-68. [Crossref] [PubMed]
- Song YS, Koo BK, Cho NH, et al. Effect of low serum total bilirubin levels (≤0.32 mg/dl) on risk of coronary artery disease in patients with metabolic syndrome. Am J Cardiol 2014;114:1695-700. [Crossref] [PubMed]
- Mahabadi AA, Lehmann N, Möhlenkamp S, et al. Association of bilirubin with coronary artery calcification and cardiovascular events in the general population without known liver disease:the Heinz Nixdorf Recall study. Clin Res Cardiol 2014;103:647-53. [Crossref] [PubMed]
- Kimm H, Yun JE, Jo J, et al. Low serum bilirubin level as an independent predictor of stroke incidence: a prospective study in Korean men and women. Stroke 2009;40:3422-7. [Crossref] [PubMed]
- Oda E, Aoyagi R, Aizawa Y. Hypobilirubinemia might be a possible risk factor of end-stage kidney disease independently of estimated glomerular filtration rate. Kidney Blood Press Res 2012;36:47-54. [Crossref] [PubMed]
- Sakoh T, Nakayama M, Tanaka S, et al. Association of serum total bilirubin with renal outcome in Japanese patients with stages 3-5 chronic kidney disease. Metabolism 2015;64:1096-102. [Crossref] [PubMed]
- Riphagen IJ, Deetman PE, Bakker SJ, et al. Bilirubin and progression of nephropathy in type 2 diabetes: a post hoc analysis of RENAAL with independent replication in IDNT. Diabetes 2014;63:2845-53. [Crossref] [PubMed]
- Okada H, Fukui M, Tanaka M, et al. Low serum bilirubin concentration is a novel risk factor for the development of albuminuria in patients with type 2 diabetes. Metabolism 2014;63:409-14. [Crossref] [PubMed]
- Mashitani T, Hayashino Y, Okamura S, et al. Correlations between serum bilirubin levels and diabetic nephropathy progression among Japanese type 2 diabetic patients:a prospective cohort study (Diabetes Distress and Care Registry at Tenri [DDCRT 5]). Diabetes Care 2014;37:252-8. [Crossref] [PubMed]
- Lee MJ, Jung CH, Kang YM, et al. Serum bilirubin as a predictor of incident metabolic syndrome: a 4-year retrospective longitudinal study of 6205 initially healthy Korean men. Diabetes Metab 2014;40:305-9. [Crossref] [PubMed]
- Huang SS, Chan WL, Leu HB, et al. Serum bilirubin levels predict future development of metabolic syndrome in healthy middle-aged non-smoking men. Am J Med 2015;128:1138.e35-41. [Crossref]
- Taylor AE, Morris RW, Fluharty ME, et al. Stratification by smoking status reveals an association of CHRNA5-A3-B4 genotype with body mass index in never smokers. PLoS Genet 2014;10:e1004799. [Crossref] [PubMed]
- Justice AE, Winkler TW, Feitosa MF, et al. Genome-wide meta-analysis of 241,258 adults accounting for smoking behaviour identifies novel loci for obesity traits. Nat Commun 2017;8:14977. [Crossref] [PubMed]
- Lee YB, Lee SE, Jun JE, et al. Change in Serum Bilirubin Level as a Predictor of Incident Metabolic Syndrome. PLoS One 2016;11:e0168253. [Crossref] [PubMed]
- Jung CH, Lee MJ, Kang YM, et al. Higher serum bilirubin level as a protective factor for the development of diabetes in healthy Korean men: A 4 year retrospective longitudinal study. Metabolism 2014;63:87-93. [Crossref] [PubMed]
Cite this article as: Oda E. Does serum bilirubin prevent cardiovascular disease? J Xiangya Med 2017;2:58.