
More complication after chronic spinal cord injury: impairment of blood flow, which could be potentially restored for functional improvement
Following a severe spinal cord injury (SCI), it is well known that spinal tissue caudal to (below) the injury site experiences major changes, such as loss of supraspinal innervations, which often results in paralysis (1). One class of neurotransmitters produced from the brain is monoamines, including serotonin [5-hydroxytryptamine (5-HT)], noradrenaline (also called norepinephrine), and dopamine, which is particularly important since they modulate not only motor, sensory, and autonomic functions of the spinal cord, but also the basal vascular tone. It is well known that spinal cord neurons below the injury undergo tremendous adaptations in order to compensate for the loss of supraspinal monoamine innervations. However, it is still a mystery whether the spinal cord vessels below injury undergo similar adaptations after SCI.
A recent study by Li et al. from Drs. Karim Fouad and David Bennett’s lab at the University of Alberta, Canada, published in Nature Medicine (2), uncovered this mystery. They found that spinal blood flow is unexpectedly impaired to a chronic ischemic level after chronic SCI and that inhibition of monoamine receptors and a key enzyme that is responsible for ischemia, or the simple increase of inhaled oxygen can substantially improve spinal blood flow, as well as motor function such as walking.
In a normal spinal cord, serotonin (5-HT) and other monoamines are produced exclusively in brainstem-derived axons (Figure 1A). That is why the two key enzymes in the 5-HT synthesis pathway, tryptophan hydroxylase (TPH) and aromatic amino acid decarboxylase (AADC), are very weakly expressed caudal to SCI due to loss of supraspinal monoamine innervations. To compensate for the loss of these enzymes from supraspinal axons, one key enzyme, AADC, is surprisingly up-regulated in blood vessel endothelial cells and pericytes, especially in pericytes, a finding from the same group, which was published in 2014 (3). Besides its involvement in synthesis of 5-HT or norepinephrine, AADC can also directly synthesize trace amines (TAs), such as tryptamine and tyramine, from endogenous amino acids like tryptophan and tyrosine (Figure 1B).
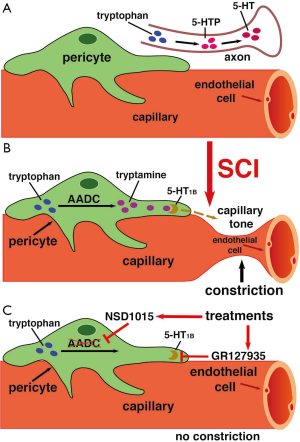
TAs at high concentration are known to activate 5-HT1 and α-adrenergic receptors, which are generally intended to be activated by monoamines like 5-HT and norepinephrine, respectively. It is known that 5-HT acting on 5-HT1 receptors, as well as norepinephrine acting on α-adrenergic receptors, causes vasoconstriction, so Li et al. hypothesized that the elevated TA levels in capillary pericytes caudal to SCI could also have an effect on vessel tone (2).
First, Li et al. confirmed the previous findings using AADC immunolabeling, observing that AADC is highly up-regulated in vessels caudal to SCI, while mostly absent in axonal tracts (2). In contrast, uninjured spinal cords showed little expression of AADC in vessels. Furthermore, double immunolabeling with pericyte markers NG2 and CD13 showed that the AADC up-regulation occurs specifically in pericytes, rather than the arterioles themselves (2). Immunolabeling for TAs showed that they are densely expressed in pericytes caudal to SCI, while immunolabeling for 5-HT revealed that it is largely absent caudal to the injury site. However, when spinal cords were treated with the 5-HT precursor 5-HTP, immunolabeling showed high expression of 5-HT, while application of an AADC inhibitor (NSD1015) eliminated 5-HT expression, confirming that 5-HT caudal to SCI is entirely produced from endogenous AADC (2).
Li et al. then demonstrated that TAs do in fact induce vasoconstriction in pericytes that wrapped around capillaries in vitro, by applying aromatic amino acids such as tryptophan, phenylalanine, and tyrosine to a spinal cord and observing that capillary diameter decreased by 25% (Figure 1B) (2). When an AADC inhibitor, NSD1015, was also applied, no vasoconstriction was observed (Figure 1C) (2), suggesting that the TAs produced from amino acids by AADC are the cause. They confirmed that the TAs were binding to 5-HT1 and α-adrenergic receptors by showing that application of the receptors’ respective inhibitors, GR127935 and RX821002, blocked vasoconstriction (Figure 1B,C) (2). In uninjured rat spinal cords, application of tryptophan had no effect, as AADC is not up-regulated in vessels before SCI.
Their study demonstrated that blood flow rates in spinal capillaries caudal to the injury site were significantly lower than normal spinal cord using a rat model, indicating that endogenous TAs induce hypoxia after SCI (2). When AADC inhibitors, such as NSD1015, or 5-HT1B receptor antagonists, such as GR127935 were applied, blood flow rates returned to normal, supporting the idea that tryptophan, through AADC, causes vasoconstriction caudal to SCI (Figure 1C) (2). Relatedly, spinal tissue caudal to the injury was chronically hypoxic, while tissue rostral to the injury had normal tissue oxygenation (pO2) levels. Again, treatment with AADC inhibitors or 5-HT1B receptor antagonists restored pO2 levels (2).
Astonishingly, placing rats in hyperoxic conditions (95–100% O2) for short periods of time enhanced blood oxygen levels for long periods of time (~20 minutes) (2). Other changes in air content, including hypoxic conditions (10% O2) and increased CO2 concentrations (10% CO2), resulted in similar increases in blood oxygenation (2). A reasonable explanation for this is that it is a positive feedback loop: the temporary increased oxygen levels stimulates neural activity, resulting in neurovascular coupling that dilates vessels, resulting in even more oxygen levels.
Accompanying the aforementioned treatments that increased blood oxygenation, spontaneous electromyography (EMG) activity in paralyzed tail muscles of conscious chronic spinal rats increased as well. These treatments also increased sensory-evoked EMG responses, called long-lasting reflexes (LLRs), and increased the incidence of rhythmic locomotor-like bursting (2). These results indicate that prevention of hypoxia caudal to SCI by blocking TAs or systemically augmenting blood pressure or transient hyperoxic breathing can enhance motor function.
Besides motor function improvement, locomotor function also improved in an incomplete thoracic contusion or a staggered-hemisection injury model after above treatments. In the new SCI model, Li et al. first confirmed that tissue is hypoxic in the lumbar region caudal to the injury site (2). Local application of GR127935 or RX821002 restored pO2 to a near normal level. Similarly, transient hyperoxic O2 breathing (95%, 90 s) also restored normal pO2. When these inhibitors were administered intrathecally, a clinically relevant approach, to dilate blood vessel capillaries, the walking ability of rats was significantly improved. Interestingly, the effect of NSD1015, an irreversible AADC inhibitor, lasted for at least 24 hours, demonstrating that locomotor function can be improved for prolonged periods of time (2).
This study has a fundamental impact on SCI research and rehabilitation since it demonstrates for the first time that pericytes impair capillary blood flow and motor function after chronic SCI, and that the treatments that can restore normal blood flow can also improve motor function. In term of rehabilitation, a simple therapy, such as exercise or a transient hyperoxic breathing, could prevent hypoxia and reduce damage to chronic SCI. This strategy, along with other rehabilitation methods, could maximize restoration of locomotion function after SCI. This is especially important for incomplete injuries, where some spared supraspinal axons can still innervate spinal cord neurons below the injury. If the spinal cord neurons below injury have a sufficient supply of blood flow, their functional activities could be easily restored or improved as demonstrated by Li et al.’s study (2).
For complete SCI, such a model as used in most of this study (S2 complete transection), a simple therapy, such as exercise or a transient hyperoxic breathing, may prevent hypoxia, but may not restore locomotor function after chronic SCI since there is no supraspinal innervation and the local spinal cord neurons alone below the injury cannot restore coordinate locomotion. On the contrary, such a simple therapy may increase muscle spasms. This is probably the reason that the authors switched the SCI model from complete transection to a thoracic SCI from either a severe contusion model or a staggered-hemisection injury, both of which retain some spared supraspinal axon innervations or supraspinal control indirectly through propriospinal relay to spinal cord neurons below the injury (4). Since most SCI in human are incomplete [America National Spinal Cord Injury Statistical Center (NSCISC)], restoration of normal blood flow below the injury could become a simple and powerful therapy for treatment of SCI.
Acknowledgments
Funding: This work was supported in part by Merit Review Award from the United States Department of Veterans Affairs.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Prof. Hongbin Lu (Department of Sports Medicine, Xiangya Hospital, Central South University, Changsha, China) and Assistant Editor Dr. Shuangfei Ni (Department of Spine Surgery, Xiangya Hospital, Central South University, Changsha, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2017.10.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rowland JW, Hawryluk GW, Kwon B, et al. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus 2008;25:E2. [Crossref] [PubMed]
- Li Y, Lucas-Osma AM, Black S, et al. Pericytes impair capillary blood flow and motor function after chronic spinal cord injury. Nat Med 2017;23:733-41. [Crossref] [PubMed]
- Li Y, Li L, Stephens MJ, et al. Synthesis, transport, and metabolism of serotonin formed from exogenously applied 5HTP after spinal cord injury in rats. J Neurophysiol 2014;111:145-63. [Crossref] [PubMed]
- Courtine G, Song B, Roy RR, et al. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med 2008;14:69-74. [Crossref] [PubMed]
Cite this article as: Wang D, Lu P. More complication after chronic spinal cord injury: impairment of blood flow, which could be potentially restored for functional improvement. J Xiangya Med 2017;2:72.


