
Office versus ambulatory blood pressure measurements in diagnosing diurnal hypertension in sub-Saharan Africans: a comparative cross-sectional study in Cameroon
Introduction
Hypertension is the most frequent cardiovascular risk factor worldwide, with the greatest burden in low-income settings (1). One-in-four to one-in-three adult in some sub-Saharan Africa (SSA) settings has hypertension (2,3). This high disease burden is associated with under-diagnosis, under-investigation, and under-treatment (4). The availability and access to diagnostic tests and medicines remain very low (5). The diagnosis of hypertension is based on persistently elevated blood pressure (BP) readings measured on two different occasions (6). The strategy used in large scale screening in epidemiologic studies of cardiovascular risk factors is a series of readings measured in a single encounter often during the daytime. It is not certain if this reflects the true prevalence rates, as white coat hypertension (WCH) has a non-negligible burden.
The aim of this cross-sectional descriptive and analytic study was to see if the serial BP readings measured in a single encounter, alongside other cardiovascular risk factors could make the diagnosis of hypertension in a group of sub-Saharan Africans. The findings will add to local evidence, and help reduce lifelong unnecessary anti-hypertensive treatment. Also, this will shed light on the true burden of hypertension in epidemiological studies carried out in our setting.
Methods
Ethical statement: this work was approved by the institutional review board of the Faculty of medicine and Biomedical Sciences, University of Yaounde 1, Cameroon. This work was carried out in accordance with the declarations of Helsinki (7). We report this work following the STROBE checklist (8).
Study design and setting: this cross-sectional descriptive and analytic comparative study was carried-out in a specialist cardiology clinic between October and November 2016, in Yaoundé—the capital city of Cameroon, SSA. The World Health Organization (WHO) STEPwise approach for epidemiological surveillance of chronic non-communicable disease was used (9). The population of the city is estimated at two million inhabitants.
Participants: these were workers in a high social standard corporation who were screened for cardiovascular risk factors. We included consenting adults aged ≥18 years of both sexes. Those with newly diagnosed elevated office (daytime) BP readings underwent a 24-hour ambulatory blood pressure measurements (ABPMs) to confirm hypertension (Test group). Known cases of hypertension on treatment that underwent ABPM to assess control of BP were excluded from the test group. We compared the clinical and biological characteristics of those with ABPM confirmed hypertension to those with normal office BP readings (Comparative group). Those with treated hypertension and normal office BP reading were excluded from the comparative group. Those with newly diagnosed elevated BP, and did not undergo ABPM were also analyzed.
Variables: participants presented to the clinic between eight and nine o’clock in the morning after an overnight fasting for at least eight hours. In WHO STEP one, we collected following data—demographics (age and sex), personal history (diabetes, hypertension, alcohol use, tobacco use, dyslipidemia, drug use), lifestyle (low risk diet, physical activity), family history (diabetes, hypertension, stroke, myocardial infarction), symptoms of vascular disease. In WHO STEP two, we carried out the following measurements—Anthropometry (height, weight, abdominal circumference, and resting BP). In WHO STEP three, we drew blood for biochemistry (glycemia, HbA1c, renal function, lipid profile, serum uric acid, electrolytes, and full blood count). We also collected urine samples from participants for dip-stick analysis.
Data sources and measurements: weight (w) was measured (kg) with an electronic scale in light clothing and with no shoes. Height (h) was measured (m) with a stadiometer close to the scalp. We calculated Body Mass Index (BMI) as w/h2. Obesity was present if the BMI was ≥30 kg/m2. We measured the abdominal circumference with a tape in the standing position mid-way between the iliac crest and the inferior costal margin, mid-axillary line. Abdominal obesity was present if this was >94 cm in men and >80 cm in women (IDF criteria). We measured their BP twice on both arms after 10 minutes of rest in the sitting position, with an electronic device (Omron®) using a standard adult arm cuff. The average of the highest recording was considered. Systolic blood pressure (SBP) readings >140 mmHg and/or diastolic blood pressure (DBP) readings >90 mmHg was considered as having elevated BP. Those with newly diagnosed elevated office BP were considered for a 24-hour ABPM. This was carried out with a GE apparatus using the oscillation method. Those with a day-time ABPM >135 mmHg (SBP) and or >85 mmHg (DBP) were considered hypertensive. Those with a high office BP and normal ABPM were considered as having WCH. Compared with the diurnal BP, those with a nocturnal drop in BP of 10% to 20% were considered as dippers, 0% to 10% as non-dippers, >20% as extreme dippers, and reverse dippers if there was an increase in the night BP. We measured HbA1c using high performance liquid chromatography (HPLC), and fasting blood glucose (FBG) using Glucose Oxidase method. A HbA1c ≥6.5% and/or FBG ≥126 mg/dL or a participant on glucose lowering medication was considered as having diabetes. Other biochemical blood tests were carried-out using standard methods. Dyslipidemia was present if a participant has at least one of the following lipid anomalies: total cholesterol >2 g/L, LDLc >1 g/L, Triglyceride >1.5 g/L, and HDLc <0.4 g/L for men and <0.5 g/L for women. Hyperuricemia was present if serum uric acid was >70 mg/L in men and >60 mg/L in women. A participant was considered a smoker if he/she used tobacco or it products within the past three years. Alcohol consumption was limited to its use (dependence was not studied).
Outcome data: the main outcome was persistent elevated diurnal BP on ABPM. Other variables studied were the components of the metabolic syndrome (10), and the 10-year cardiovascular risks (risk estimated using the Framingham risk score calculator). The 10-year cardiovascular risk is the probability of having a heart attack, stroke, or other vascular events within 10 years. This risk depends on the age, sex, BPs, cholesterol levels, diabetes, and smoking status. The risk is said to be low (<1%), moderate (1–5%), high (5–10%), and very high (>10%). We also looked at the hyperuricemia and high risk life-style.
Study size: this was a cross-sectional descriptive and analytic study involving a group of workers in SSA. A convenient sample of all eligible participants was considered for this study.
Statistical methods: we analyzed the data using Epi-Info version 7. We present the baseline characteristics according to test group versus comparative group. We have presented discrete variables as frequencies and proportions with their 95% confidence intervals. We have presented continuous variables as means ± standard deviations. Differences between proportions were compared using Chi-square or Fischer exact tests where applicable. Differences between mean values were compared using two ways ANOVA or Kruskal-Wallis test where applicable. A P value <0.05 was considered statistically significant for the observed differences.
Results
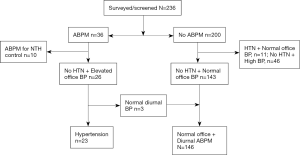
Participants: the flow of the participants is shown in Figure 1. A total of 236 participants underwent screening. Of these, 36 had an ABPM performed, of which 10 were excluded—because they were known cases of hypertension on treatment—and 26 were analyzed (test group). Of the 200 participants who did not have an ABPM, 57 were excluded—11 were known hypertensives on treatment with normal office BP, and 46 had de novo raised office BP but did not come for ABPM. The 143 participants with normal office BP and no personal history of hypertension served as the comparative group.
Descriptive data: the clinical and biochemical characteristics of the study participants (diurnal hypertension versus no diurnal hypertension) are shown in Table 1. Those with diurnal hypertension were significantly older (50.3 vs. 41.7 years), had higher adiposity (99.2 vs. 89.6 cm of abdominal circumference), pulse pressure (66.7 vs. 49.2 mmHg), fasting blood glucose (0.89 vs. 0.82 g/L), total cholesterol (2.16 vs. 1.95 g/L), triglycerides (1.38 vs. 0.69 g/L), and serum uric acid (67.1 vs. 54.8 mg/L). The mean 10-year Framingham risk score was significantly higher in the test group (21.4% vs. 6.8%).
Table 1
| Characteristics | Diurnal hypertension | Normal office + diurnal ABPM | P value |
|---|---|---|---|
| Age (years), mean (SD) | 50.3 (7.2) | 41.7 (10.2) | <0.001 |
| Male sex, % | 15 (65.2) | 80 (54.8) | 0.352 |
| Personal history, % | |||
| Diabetes | 2 (8.7) | 3 (2.1) | 0.085 |
| Dyslipidemia | 1 (4.3) | 7 (4.8) | 0.917 |
| Tobacco use | 3 (13.0) | 14 (9.6) | 0.616 |
| Alcohol use | 14 (60.9) | 84 (57.5) | 0.544 |
| Physical activity | |||
| None | 5 (21.7) | 47 (32.2) | 0.312 |
| Less than 3 times/week | 14 (60.9) | 79 (54.1) | 0.544 |
| More than 3 times/week | 4 (17.4) | 20 (13.7) | 0.638 |
| No risk reducing diet | 18 (81.8) | 131 (91.6) | 0.142 |
| Family history, % | |||
| Stroke | 5 (21.7) | 22 (15.1) | 0.424 |
| Heart attack | 1 (4.3) | 3 (2.1) | 0.523 |
| Hypertension | 8 (34.8) | 56 (38.4) | 0.742 |
| Diabetes | 3 (13.0) | 46 (31.5) | 0.069 |
| Complaints, % | |||
| Chest pain | 2 (8.7) | 10 (6.8) | 0.742 |
| Palpitation | 0 (0) | 28 (19.2) | 0.022 |
| Dyspnoea | 1 (4.3) | 11 (7.5) | 0.579 |
| Orthopnea | 0 (0) | 2 (1.4) | 0.569 |
| Snoring | 3 (13) | 32 (21.9) | 0.329 |
| Headaches | 2 (8.7) | 4 (2.7) | 0.148 |
| Physical findings, mean (SD) | |||
| Pulse pressure (mmHg), mean (SD) | 66.7 (18.9) | 49.2 (9) | <0.001 |
| Heart rate, mean (SD) | 73.5 (12.2) | 69.8 (9.5) | 0.098 |
| BMI (kg/m2), mean (SD) | 30.1 (3.3) | 27.3 (4.3) | 0.003 |
| Abdominal circumference (cm), mean (SD) | 99.2 (6.8) | 89.6 (10.9) | <0.001 |
| Heart murmurs, % | 1 (4.3) | 5 (3.4) | 0.828 |
| Blood chemistry | |||
| FBG (g/L), mean (SD) | 0.89 (0.1) | 0.82 (0.07) | 0.006 |
| HbA1c (%), mean (SD) | 5.4 (0.7) | 5.2 (0.66) | 0.172 |
| Total cholesterol (g/L), mean (SD) | 2.16 (0.5) | 1.95 (0.4) | 0.023 |
| HDLc (g/L), Mean (SD) | 0.63 (0.18) | 0.63 (0.19) | 0.897 |
| Triglycerides (g/L), mean (SD) | 1.38 (2.3) | 0.69 (0.4) | 0.001 |
| LDLc (g/L), mean (SD) | 1.33 (0.45) | 1.17 (0.39) | 0.067 |
| Total cholesterol/HDLc ratio | 3.7 (1.2) | 3.2 (1.0) | 0.071 |
| Serum uric acid (mg/L), mean (SD) | 67.1 (14.2) | 54.8 (19.4) | 0.004 |
| Serum creatinine (mg/L), mean (SD) | 10.1 (2.4) | 8.98 (2.97) | 0.091 |
| 10-year vascular risk (%), mean (SD) | 21.4 (10.8) | 6.8 (6.2) | <0.001 |
ABPM, ambulatory blood pressure measurement; BMI, body mass index; FBG, fasting blood glucose.
Outcome data and main results: of the 26 participants who had an ABPM indicated for de novo raised office BP, 23 (88.5%) had hypertension, and 3 (11.5%) had normal BP-WCH. Of the participants with hypertension (n=22), 6 (27.3%) were dippers, 2 (9.1%) were extreme dippers, 9 (40.9%) were non-dippers, and 5 (22.7%) were reverse dippers or night speakers. The three participants with white coat effect had a non-dipping pattern. The determinants of de novo hypertension on ABPM are shown in Table 2. Age >50 years (OR: 4.1, P=0.001), adiposity (OR: 6.2, P=0.002), pulse pressure >65 mmHg (OR: 21.4, P<0.001), and sex specific hyperuricemia (OR: 4.2, P=0.001) were predictors of de novo hypertension on ABPM. After adjusting for age: adiposity, pulse pressure, and hyperuricemia were still associated with de novo diurnal hypertension on ABPM (Table 3). These determinants were not associated with the non-dipping or reverse dipping pattern (Table S1).
Table 2
| Variables | Diurnal hypertension (n=23) | No diurnal hypertension (n=146) | OR (95% CI) | P value |
|---|---|---|---|---|
| Age >50 years | 14 (60.9) | 40 (27.4) | 4.1 (1.7–10.3) | 0.001 |
| Overweight + obesity | 22 (95.7) | 106 (72.6) | 8.3 (1.1–63.7) | 0.017 |
| Obesity | 10 (43.5) | 41 (28.1) | 1.97 (0.8–4.8) | 0.134 |
| Abdominal obesity | 19 (86.4) | 73 (50.7) | 6.2 (1.8–21.7) | 0.002 |
| Pulse pressure >65 mmHg | 11 (47.8) | 4 (4.1) | 21.4 (6.7–67.9) | <0.001 |
| Fasting blood glucose >1 g/L | 3 (13.0) | 5 (3.4) | 4.2 (0.9–19.1) | 0.044 |
| HbA1c >5.7% | 9 (39.1) | 33 (22.6) | 2.2 (0.87–5.5) | 0.088 |
| Hyperuricemia | 11 (47.8) | 26 (17.8) | 4.2 (1.7–10.6) | 0.001 |
| Total cholesterol >2 g/L | 14 (60.9) | 68 (46.6) | 1.8 (0.7–4.4) | 0.202 |
| Triglycerides >1.5 g/L | 3 (13.0) | 6 (4.1) | 3.5 (0.8–15.1) | 0.076 |
| Total cholesterol/HDLc ratio >4.5 | 6 (26.1) | 17 (11.6) | 2.7 (0.9–7.7) | 0.06 |
Table 3
| Variables | aOR (95% CI) | P value |
|---|---|---|
| Overweight + obesity | 7.1 (0.96–52.4) | 0.017 |
| Obesity | 1.97 (0.8–5.0) | 0.113 |
| Abdominal obesity | 5.6 (1.6–19.7) | 0.002 |
| Pulse pressure >65 mmHg | 19.6 (5.6–68.0) | <0.001 |
| Fasting blood glucose >1 g/L | 4.2 (0.8–22.6) | 0.126 |
| HbA1c >5.7% | 1.85 (0.7–4.8) | 0.154 |
| Hyperuricemia | 3.8 (1.5–9.8) | 0.006 |
| Total cholesterol >2 g/L | 1.4 (0.5–3.5) | 0.331 |
| Triglycerides >1.5 g/L | 2.9 (0.5–12.3) | 0.196 |
| Total cholesterol/HDLc ratio >4.5 | 3.1 (0.99–9.1) | 0.056 |
Other analyses: the clinical and biochemical characteristics of those with high office BP, and those without ABPM were similar (Table S2).
Discussion
We carried out this cross-sectional descriptive and analytic comparative study with the aim of assessing the diagnoses of hypertension from serial BP measures in a single encounter, compared with ABPM-standard. We also sought to study the determinants of de novo hypertension on ABPM. Eighty nine percent (89%) of those with high office BP had hypertension on ABPM, and 11% had WCH. Older age, adiposity, high pulse pressure, and hyperuricemia were associated with diurnal hypertension on ABPM, but seem not to be associated with the BP dipping pattern.
This study should be interpreted in the light of some limitations. We had a small sample size due to the restricted population of participants, thus reducing our ability to detect significant differences or associations. Many participants who had an indication for ABPM did not come for the test. However, their clinical and biochemical characteristics were similar with those who had the test done. Thus, our findings could be extrapolated to these groups. Those with normal office BP could have masked hypertension which we could not detect with our study design. Despite these limitations, this study sheds light on the burden of true hypertension in our setting.
The strategy of serial BP measurements in a single encounter during the day to diagnose hypertension is often used in epidemiological studies. With this strategy, the prevalence of hypertension could be over-estimates due to the additional burden of White coat effect, or under-estimates due to the hidden burden of masked hypertension. Also, the hemodynamic changes-dipping pattern—that occur during night time sleep are not captured using this approach. This makes ABPM a better screening tool (11). However, this technology is not widely available in low—income settings especially in SSA. We found that close to 9-in-10 patients with de novo raised office BP had true hypertension on ABPM, and this was associated with age, adiposity, and hyperuricemia. Taka et al. (12) found twice as much cases of WCH in a large Cameroon cohort, which was independently associated with BMI only. Zhou et al. (13) reported 14% of WCH in a group of diabetic patients in China, and Dolan et al. (14) reported 15% of WCH in a large cohort of patients similar ours in Ireland. Pengkeaw et al. (15) reported up to 45.2% of WCH in a small cohort of patients in Thailand. This difference could be due to the selection of the participants as suggested by Verdecchia et al. (16). The determinants of WCH were not consistent between the studies. In our study, global obesity and or overweight were not significantly associated with true diurnal hypertension unlike abdominal obesity. This suggests that the true prevalence of hypertension is modulated by the presence of other vascular risk factors. We did not find any association of the determinants of hypertension and the dipping pattern—probably due to the low statistical power of our study. This needs to be studied further in a larger sample of participants. The three participants with WCH all had a non-dipper pattern. The small number of participants did not permit us carryout further analysis. However, this was similarly reported by Zhou et al. (13), who found up to 35% of non-dippers and a further 13% of reverse dippers in a Chinese cohort with type 2 diabetes and WCH. This suggests that WCH could be a reflection of an altered hemodynamics with a possible long-term adverse outcome. This hypothesis needs to be studied further in our setting, as the clinical significance of WCH is uncertain as reported by Verdecchia et al. (16). However, the recent meta-analysis by Huang et al. (17) showed that WCH is also a cardiovascular risk factor compared with normotensives. The blunted nocturnal dipping and even reverse dipping as reported by Zhou et al. (13), strengthened by our finding—despite the small sample—supports the findings of Huang et al. (17). Lande et al. (18) showed that WCH could be associated with the end-organ damage effect of sustained hypertension in children. However, the paucity of data on APBM in our setting calls for further studies to assess the predictors of the different patterns of BP in our setting.
Conclusions
In people with de novo raised office BP, 9-in-10 will have true diurnal hypertension on ABPM especially when they are aged >50 years, have abdominal obesity, high pulse pressure, and hyperuricemia. Those with White coat effect appeared to have a blunted BP dipping pattern.
Table S1
| Variable | Non dipper | Dipper | OR (95% CI) | P value |
|---|---|---|---|---|
| Age >50 years | 10 (71.4) | 3 (37.5) | 4.2 (0.7–26.3) | 0.135 |
| Overweight + obesity | 13 (92.9) | 8 (100.0) | - | - |
| Obesity | 3 (21.4) | 6 (75.0) | 0.09 (0.01–0.72) | 0.021 |
| Abdominal obesity | 11 (84.6) | 7 (87.5) | 0.79 (0.06–10.4) | 0.684 |
| Pulse pressure >65 mmHg | 8 (57.1) | 3 (37.5) | 2.2 (0.38–13.2) | 0.329 |
| Fasting blood glucose >1 g/L | 3 (17.6) | 0 (0.0) | - | - |
| HbA1c >5.7% | 5 (35.7) | 4 (50) | 0.56 (0.1–3.2) | 0.416 |
| Hyperuricemia | 6 (42.9) | 5 (62.5) | 0.45 (0.1–2.7) | 0.329 |
| Total cholesterol >2 g/L | 9 (64.3) | 4 (50) | 1.83 (0.3–10.5) | 0.416 |
| Triglycerides >1.5 g/L | 3 (17.6) | 0 (0.0) | - | - |
| Total cholesterol/HDLc ratio >4.5 | 6 (35.3) | 0 (0.0) | - | - |
ABPM, ambulatory blood pressure measurement.
Table S2
| Characteristics | ABPM (n=26) | No ABPM (n=46) | P value |
|---|---|---|---|
| Age (years), mean (SD) | 50.3 (7.2) | 50.5 (9.5) | 0.926 |
| Male sex, % | 15 (65.2) | 26 (56.5) | 0.473 |
| Physical findings, mean (SD) | |||
| Pulse pressure (mmHg), mean (SD) | 66.7 (18.9) | 65.8 (13.8) | 0.817 |
| Heart rate, mean (SD) | 73.5 (12.2) | 74.7 (12.1) | 0.619 |
| BMI (kg/m2), mean (SD) | 30.1 (3.3) | 31 (5.3) | 0.437 |
| Abdominal circumference (cm), mean (SD) | 99.2 (6.8) | 100.5 (12.7) | 0.630 |
| Blood chemistry | |||
| FBG (g/L), mean (SD) | 0.89 (0.1) | 0.94 (0.3) | 0.414 |
| HbA1c (%), mean (SD) | 5.4 (0.7) | 5.7 (1.5) | 0.340 |
| Total cholesterol (g/L), mean (SD) | 2.16 (0.5) | 2.02 (0.5) | 0.256 |
| HDLc (g/L), mean (SD) | 0.63 (0.18) | 0.59 (0.2) | 0.401 |
| Triglycerides (g/L), mean (SD) | 1.38 (2.3) | 0.88 (0.56) | 0.163 |
| LDLc (g/L), mean (SD) | 1.33 (0.45) | 1.24 (0.4) | 0.384 |
| Total Cholesterol/HDLc ratio | 3.7 (1.2) | 3.7 (1.2) | 1 |
| Serum Uric acid (mg/L), mean (SD) | 67.1 (14.2) | 62.2 (14.4) | 0.168 |
| Serum Creatinine (mg/L), mean (SD) | 10.1 (2.4) | 9.97 (2.8) | 0.843 |
| 10-year vascular risk (%), mean (SD) | 21.4 (10.8) | 18.5 (9.88) | 0.251 |
ABPM, ambulatory blood pressure measurement; BMI, body mass index; FBG, fasting blood glucose.
Acknowledgments
We thank the support staff of centre Medical de Hippodrome (CMH)–Yaoundé, for assisting with patient care.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2017.11.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This work was approved by the institutional review board of the Faculty of medicine and Biomedical Sciences, University of Yaoundé 1, Cameroon. This work was carried out in accordance with the declarations of Helsinki (as revised in 2013). We report this work following the STROBE checklist. Informed consent was taken from all subjects.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet 2005;365:217-23. [Crossref] [PubMed]
- Kingue S, Ngoe CN, Menanga AP, et al. Prevalence and Risk Factors of Hypertension in Urban Areas of Cameroon: A Nationwide Population-Based Cross-Sectional Study. J Clin Hypertens (Greenwich) 2015;17:819-24. [Crossref] [PubMed]
- Dzudie A, Kengne AP, Muna WF, et al. Prevalence, awareness, treatment and control of hypertension in a self-selected sub-Saharan African urban population: a cross-sectional study. BMJ Open 2012;2:e001217. [Crossref] [PubMed]
- Noubiap JJ, Jingi AM, Veigne SW, et al. Approach to hypertension among primary care physicians in the West Region of Cameroon: substantial room for improvement. Cardiovasc Diagn Ther 2014;4:357-64. [PubMed]
- Jingi AM, Noubiap JJ, Ewane Onana A, et al. Access to diagnostic tests and essential medicines for cardiovascular diseases and diabetes care: cost, availability and affordability in the West Region of Cameroon. PLoS One 2014;9:e111812. [Crossref] [PubMed]
- Krause T, Lovibond K, Caulfield M, et al. Management of hypertension: summary of NICE guidance. BMJ 2011;343:d4891. [Crossref] [PubMed]
- World Medical Association declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA 1997;277:925-6. [Crossref] [PubMed]
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495-9. [Crossref] [PubMed]
- Riley L, Guthold R, Cowan M, et al. The World Health Organization STEPwise Approach to Noncommunicable Disease Risk-Factor Surveillance: Methods, Challenges, and Opportunities. Am J Public Health 2016;106:74-8. [Crossref] [PubMed]
- Azantsa BGK, Ntentié RF, Mbong MA, et al. Body Mass Index, Blood Pressure and Hypertension Subtypes among Untreated Hypertensive Cameroonians. Br J Med Med Res 2013;3:2119-31. [Crossref]
- Staessen JA, Beilin L, Parati G, et al. Task force IV: Clinical use of ambulatory blood pressure monitoring. Participants of the 1999 Consensus Conference on Ambulatory Blood Pressure Monitoring. Blood Press Monit 1999;4:319-31. [PubMed]
- Takah N, Dzudie A, Ndjebet J, et al. Ambulatory blood pressure measurement in the main cities of Cameroon: prevalence of masked and white coat hypertension, and influence of body mass index. Pan Afr Med J 2014;19:240. [Crossref] [PubMed]
- Zhou J, Liu C, Shan P, et al. Characteristics of white coat hypertension in Chinese Han patients with type 2 diabetes mellitus. Clin Exp Hypertens 2014;36:321-5. [Crossref] [PubMed]
- Dolan E, Stanton A, Atkins N, et al. Determinants of white-coat hypertension. Blood Press Monit 2004;9:307-9. [Crossref] [PubMed]
- Pengkeaw P, Suwannakarn S. Prevalence of hypertension in suspected hypertensive patients in Rajavithi Hospital using ambulatory blood pressure monitoring. J Med Assoc Thai 2014;97:S25-30. [PubMed]
- Verdecchia P, Angeli F, Gattobigio R, et al. The clinical significance of white-coat and masked hypertension. Blood Press Monit 2007;12:387-9. [Crossref] [PubMed]
- Huang Y, Huang W, Mai W, et al. White-coat hypertension is a risk factor for cardiovascular diseases and total mortality. J Hypertens 2017;35:677-88. [Crossref] [PubMed]
- Lande MB, Meagher CC, Fisher SG, et al. Left ventricular mass index in children with white coat hypertension. J Pediatr 2008;153:50-4. [Crossref] [PubMed]
Cite this article as: Jingi AM, Kuate-Mfeukeu L, Amougou SN, Hamadou B, Nganou CN, Ateba NA, Wawo EG, Kingue S. Office versus ambulatory blood pressure measurements in diagnosing diurnal hypertension in sub-Saharan Africans: a comparative cross-sectional study in Cameroon. J Xiangya Med 2017;2:74.


