
Baffle or switch, ring or no ring: cardiac computed tomography unbaffles a complex congenital heart disease case
Introduction
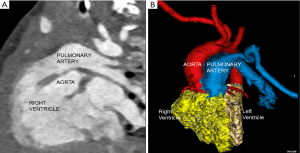
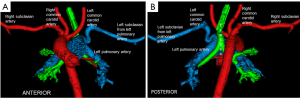
A male infant born at 39 weeks of gestation presented with a fetal diagnosis of bladder exstrophy and double outlet right ventricle (DORV). Echocardiogram revealed a large subpulmonary conoventricular septal defect. Both great arteries arose from the right ventricle suggesting a diagnosis of DORV. Right aortic arch with a large patent right ductus arteriosus (PDA) was seen with suspicion of an aberrant left subclavian artery (LSCA). The coronary artery anatomy was also not well delineated. Due to the location of the ventricular septal defect (VSD), uncertain coronary artery origin and aortic arch branching pattern, an electrocardiogram (ECG)-gated computed tomography (CT) with dose modulation was performed for planning appropriate surgical procedure and timing of surgery. The CT scan confirmed the diagnosis of DORV but showed a subaortic location of VSD (Figure 1). The LSCA arose from the proximal segment of the left pulmonary artery with normal coronary artery origin and a right sided PDA (Figure 2). The child was initially managed expectantly. However due to worsening pulmonary over circulation and need of increasing medical therapy it was decided to proceed with surgical palliation. After multi-disciplinary meeting among cardiologists, anesthesiologists and cardio-thoracic-surgeons, it was decided that because of the recent upper respiratory infections as well as bladder exstrophy the best approach would be to proceed with surgical palliation consisting of a pulmonary artery banding as opposed to a complete repair which would carry a greater surgical risk. The child underwent palliative pulmonary artery banding and is awaiting corrective surgery.
Discussion
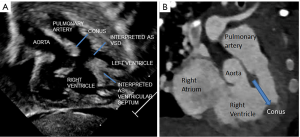
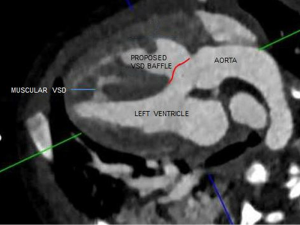
An isolated or anomalous LSCA is a rare entity. It is seen in association with other congenital heart diseases and chromosomal aberrations like 22q11 deletion. This vascular malformation does not constitute a complete vascular ring, especially with a PDA that is ipsilateral to the arch sidedness (1). Embryologically, there is involution of the aortic arch at two segments: between the left subclavian and left common carotid artery and distal to the right ductus and right subclavian artery. The subclavian artery is supplied by either the pulmonary (via ductus) artery or the vertebral artery (2). In our case the VSD was thought to be subpulmonary on the echocardiogram due to the location of the conal septum and the right ventricular papillary muscle which gave the appearance of a subpulmonary VSD, when indeed this was the right ventricular outflow tract (Figure 3). Multiplanar three-dimensional (3D)-CT provided accurate information about the routability of the VSD and showed that a baffle could be constructed from the left ventricle to the aorta via the VSD without any obstruction (Figure 4). The decision to employ CT angiography (CTA) instead of magnetic resonance angiography was taken due to underlying critical condition of the child. CTA was thus indispensable in our patient’s context for providing a timely and accurate diagnosis without the need for long-term sedation. CTA also avoided an invasive cardiac catheterization for diagnostic purposes.
Hemodynamics
The utility of CT also lies in that the available data set could also be employed for advanced post-processing techniques to generate hemodynamics information using computational modelling (3-5). Computational flow dynamics (CFD) can be generated from several commercial available software. Computational fluid dynamics would provide some insights into the flow patterns in the unusual arterial anatomy. The data may help in surgical planning of univentricular versus biventricular surgery. CFD analysis may provide hemodynamic data by the virtually created surgical baffles.
3D printing
The CT data sets also provide option for producing patient-specific 3D anatomical models especially in complex congenital heart diseases as ours. Recently, virtual 3D holographic images have also been suggested as an alternative to physical 3D models. Both anatomical and holographic 3D models may improve surgical outcomes with decrease in intra-operative complication (6,7). 3D models are likely to give impetus to better teaching in the academic world.
Our case provides indispensable utility of 3D-CT in diagnosing complex congenital heart disease and in planning the right surgical procedure and timing with an added option for providing 3D models and hemodynamics data if needed.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2018.05.03). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). As this was a reflection case report without any identifiers, thus board approval and patient informed consent are not required.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McVadon D, Shakti D, Knudson J, et al. Isolated Left Subclavian Artery From the Pulmonary Artery Masked by Pulmonary Hypertension. World J Pediatr Congenit Heart Surg 2016;7:765-8. [Crossref] [PubMed]
- Hanneman K, Newman B, Chan F. Congenital Variants and Anomalies of the Aortic Arch. Radiographics 2017;37:32-51. [Crossref] [PubMed]
- DeCampli WM, Argueta-Morales IR, Divo E, et al. Computational fluid dynamics in congenital heart disease. Cardiol Young 2012;22:800-8. [Crossref] [PubMed]
- Marsden AL, Feinstein JA. Computational modeling and engineering in pediatric and congenital heart disease. Curr Opin Pediatr 2015;27:587-96. [Crossref] [PubMed]
- Karmonik C, Partovi S, Rengier F, et al. Hemodynamic assessment of partial mechanical circulatory support: data derived from computed tomography angiographic images and computational fluid dynamics. Cardiovasc Diagn Ther 2015;5:160-5. [PubMed]
- Meier LM, Meineri M, Qua Hiansen J, et al. Structural and congenital heart disease interventions: the role of three-dimensional printing. Neth Heart J 2017;25:65-75. [Crossref] [PubMed]
- Otton JM, Birbara NS, Hussain T, et al. 3D printing from cardiovascular CT: a practical guide and review. Cardiovasc Diagn Ther 2017;7:507-26. [Crossref] [PubMed]
Cite this article as: Sarv P, Natasha GEK, Simon KC, Ravi AC. Baffle or switch, ring or no ring: cardiac computed tomography unbaffles a complex congenital heart disease case. J Xiangya Med 2018;3:22.


