
Use of cardiopulmonary bypass in lung cancer surgery: focus on extended pulmonary resections for T4 non-small cell lung cancer
Introduction
Utilization of CPB for resection of primary and secondary tumors of the heart is well established (1). Most primary cardiac tumors are benign (75%) of which 50% are myxomas and represent the most common cardiac tumor. The most common primary malignant cardiac tumor is sarcoma while the more common metastatic or secondary malignant cardiac tumors to the heart or pericardium occur in 10% of patients with other primary malignancies (2,3). Various other vascular structures such as the pulmonary arteries and major cardiac veins have also required resection for such malignancies (4). A review of the resection of locally advanced lung cancer involving these structures was extensively reviewed by Toomes and Vogt-Moykpof in 1985 (5). The use of CPB for such resection was reported as early as 1987 and a more comprehensive series was reported by Ricci et al. in 1994 (6,7).
Surgery for NSCLC is routinely performed nowadays in many countries using various techniques. Minimally invasive approaches including video assisted thoracic surgery (VATS) and robotic assisted thoracic surgery (RATS) have become quite popular. However, in cases where the primary tumor is extensive adequate exposure may require various extended incisions and adjunctive techniques. One facet is the occasional requirement of CPB for various complex situations. Usually, the tumor is an advanced primary stage T4 lesion which invades a major vascular, cardiac or airway structure. There are occasions, however, when there exists combined cardiac disease and lung cancer which may both be best managed by surgery and either requires or may benefit from a simultaneous approach. Finally, there is the rare instance when intraoperative injury to a major vascular structure during lung cancer resection can be best managed using the aid of CPB if available. Currently, alternatives to utilization of CPB such as endovascular stenting and off pump coronary artery bypass grafting (CABG) have been useful even in these complex situations. These topics will also be discussed in some detail.
The authors have a collective experience of over 50 years of either cardiac and/or lung cancer surgery and have had the occasion to employ the combined techniques of CPB during lung cancer resection. However, instead of presenting personal data this chapter will review the literature and focus mainly on scenarios involving advanced stage T4 tumors. There will be brief discussions of some of the other situations mentioned above.
T4 tumors
Much has been written about the feasibility of resection for T4 lung cancers. The AJCC 8th edition definition of a T4 stage lung cancer includes tumor >7 cm in greatest dimension, separate tumor nodules in different ipsilateral lobes or invasion of either diaphragm, mediastinum, heart, great vessels, trachea, recurrent laryngeal nerve, esophagus, vertebral body or carina (8). Often times these structures can be technically sacrificed with reasonable safety. A recent single institution study has shown an overall 5-year survival of 40% for T4 NSCLC (9). In this series of 375 patients of which 20 required CPB for resection there was no difference without or with CPB in overall survival (40% vs. 37%, respectively) or disease free survival (33% for both). Ten of the 20 patients in the CPB group had either aortic (5) or pulmonary artery trunk (7) resection.
In a recent review covering a 20-year period from 1990 to 2010, 72 patients of which 82% had T4 disease required CPB for resection (10). Overall survival was remarkably similar at 37%. However, in patients who underwent a planned CPB as opposed to an unplanned intervention the overall survival was 54%. A multivariate analysis demonstrated that unplanned or emergent use of CPB was prognostic for worse overall survival at 5 years (11%). Although not stated one would surmise that early complications and mortality was at least partially responsible for poor late survival.
In the review series reported by Muralidaran it is interesting that the lymph node status (N0 vs. N1–2) was not a determinant of late outcomes. This was thought possibly due to small sample size. However, others have seen a more conventional relationship between lymph node status and late survival. Yang et al. have shown in a multivariate analysis of 146 patients that factors effecting overall survival in the surgical treatment of T4 NSCLC are lymph node status (N2 positive, RR 3.22, P<0.001), the adequacy of resection (R0 resection, RR 0.387, P=0.002) and the structures involved (pulmonary arterial trunk 0.365, P<0.001) (11).
In two recent single institutional series and one literature review there was no difference in the 30-day mortality between patients undergoing resection with the aid of CPB and those who were resected without the use of CPB (9,10,12). In the series review by Mulralidaran the 30- and 90-day mortality was 0% and 1%, respectively. When comparison was made there was no difference in the major postoperative complication rate including respiratory failure and no difference in the median length of hospital stay (9). Since most other series are small and may represent selective results or include extensive resection without the use of CPB it is difficult to definitively validate these results.
At our institution patients routinely undergo chest computed tomography (CT) scans with contrast and positron emission tomography (PET) scans to aide in determining the overall clinical stage. Often, we will obtain an MRI scan or a CT angiogram (CTA) to improve the accuracy of staging the primary tumor. Routine bronchoscopy including endobronchial ultrasound (EBUS) is employed to further stage advanced tumors. In addition, those patients who need CPB undergo a complete cardiac evaluation including cardiology consultation, echocardiography, nuclear stress test and a left heart cardiac catheterization if necessary.
Vascular structures
Invasion of the heart, major associated venous structures, aorta and great vessels present concerns for bleeding and satisfactory maintenance of circulation both of which can be addressed by the use of CPB. Several studies have shown that surgical resection of some T4 tumors that involve these structures can be performed without the use of CPB (11,13). The major concern of use of CPB at the time of lung cancer resection is threefold. First, the associated suppression of the immune response by CPB has been well documented. However, no clear connection of this phenomenon to increase risk of cancer related mortality has ever been demonstrated. Second, the use of CPB and required anticoagulation has raised concern of systemic spread of cancer cells during resection as shed blood is scavenged and returned to the patient during surgical resection. Again, there has been no clear evidence to support this hypothesis. In fairness, the small number of such cases utilizing CPB during resection and the advanced stage of the disease make outcomes analysis difficult to interpret. One other concern is that of respiratory failure in the perioperative period. Indeed, with the improvement in CPB technology and the relatively brief time on CPB required in most of these situations lung injury should be minimal.
On the contrary, the use of CPB does increase the safety of resection particularly for tumors invading major vascular structures. A recent systematic review of studies over a 20-year period, support that in the elective setting overall 5-year survival was 54% (10). Involved structures included the aorta, left atrium, pulmonary veins, pulmonary artery, superior vena cava (SVC), inferior vena cava and right atrium.
Some have argued that the use of extracorporeal membrane oxygenation (ECMO) when possible may mitigate the above mentioned negative impact of CPB (14). Firstly, in a closed ECMO circuit scavenging of shed blood is avoided. Secondly, circulating blood through the ECMO circuit may lessen the proinflammatory response seen with CPB and possibly indirectly lessen the concern of immune suppression. Lastly, decreasing the anticoagulation requirement with ECMO over CPB may lessen the concern of bleeding and lung hemorrhage. Although most of these arguments are theoretical ECMO may be more attractive to utilize in the appropriate setting.
Although over 80% of our lobectomies at our institution are performed by minimally invasive techniques (VATS or RATS) the use of CPB requires a posterolateral thoracotomy, median sternotomy or clamshell approach. Femoral arterial and venous cannulation can be combined with any of the open approaches if the appropriate groin region has been surgically prepared ahead of time. Improvement in venous return can be obtained by bi-caval cannulation if appropriate or by the use of intra-cardiac vents either on the right or left side depending on the particular situation. This latter maneuver can also enhance exposure by creating a “bloodless” field.
In situations where cardioplegic diastolic arrest is necessary when dealing with left sided cardiac structures such as the left atrium standard moderate core temperature cooling to 34 degree centigrade with aortic cross clamping is employed. Either antegrade or retrograde infusion of cardioplegia can be performed depending on the exposure. Consideration for adequate de-airing at the conclusion of the procedure can be obtained by placement of left sided intra-cardiac vent. Lastly, if circulatory arrest is required which is rarely the case standard core cooling to 26–28 degrees centigrade and cardioplegic diastolic arrest can be considered. Such a situation could occur if tumor has invaded the aortic arch or great vessels and resection of these structures is necessary to successfully remove the lesion.
Several anesthetic considerations are important in the management of patients undergoing combined lung resection and CPB. In addition to the preoperative evaluation mentioned above the patient should have an anesthesiology consultation. Intraoperatively, cardiovascular monitoring with pulmonary artery catheter may not be prudent for planned resections of the SVC or pulmonary artery trunk. Transesophageal echocardiography (TEE) is invaluable in monitoring biventricular function and pulmonary arterial pressures particularly when pneumonectomy is planned. It will also allow evaluation of intracardiac pathology and monitoring of residual intracardiac air if left sided cardiac structures are such as the left atrium are manipulated. Judicious fluid management to limit lung edema should include avoidance of excessive fluid administration, retrograde pump priming to limit hemodilution, ultrafiltration and diuresis (15). Finally, pain management should not include preoperative placement of epidural catheters due to required anticoagulation but may be placed in the postoperative period after normalization of coagulation. Adjuncts such as placement of paravertebral or intercostal nerve blocks and postoperative use of IV patient controlled analgesia should be employed.
Two areas of particular concern which are reported at a higher frequency are T4 tumors involving the pulmonary veins or left atrium and those tumors invading the aorta. In the former situation there is concern of leaving residual tumor/clot within the left atrium without the use of CPB support and cardioplegic arrest. If preoperative studies demonstrate significant intra-atrial disease then controlled surgical resection in a bloodless field with atrial wall reconstruction is optimal (Figure 1). Certainly, radiographic or echocardiographic evidence of tumor reaching to the level of the mitral valve makes the use of CPB imperative. This technique ensures complete tumor resection and minimizes the risk of tumor embolization. Literature controversy regarding the use of CPB in this setting is difficult to reconcile as the degree of intra-atrial involvement cannot be determined in most reports. Although there are several reports of performing resection of left atrial tumors without the use of CPB a recent case report from Toronto General Hospital highlights the issue of the degree atrial involvement (15). In this report a patient underwent attempted resection without CPB unsuccessfully and required transfer to the medical center for successful resection with CPB.
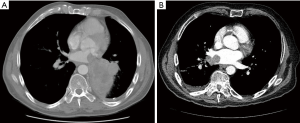
The laterality of reported resections is equivalent. It is difficult to determine from the literature whether T4 lesions involving the inferior or superior pulmonary vein are more likely to involve surrounding major structures. However, several reports indicate that T4 lesions of the left lung that involve the LA may also involve the pulmonary artery or aorta while T4 lesions of the right lung that involve the LA may also involve the right atrium (RA) or SVC (9,12,16,17). These more complex cases further the need for CPB support during resection.
A second area of concern is tumor invasion of the aorta. Radiographic evidence by either CT or magnetic resonance imaging (MRI) of obliteration of the fat plane between the tumor and the aortic wall, effacement of the aortic wall, or actual intraluminal thrombus/tumor makes this likely a T4 lung cancer (Figure 2). Others have also used contact between the tumor and the aortic wall extending for greater than 3 cm or surrounding more than 90 degrees of the circumference (18). Symptoms are relatively rare but evidence of distal emboli such as the visceral ischemia or the “blue toe” syndrome increases the likelihood of aortic wall penetration. In these situations depending on the location of the aortic involvement various techniques can be utilized to achieve an R0 resection including CPB, aorto-aortic shunt, direct clamping only and endografting.
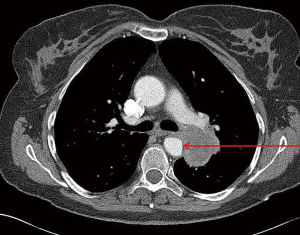
In general, endovascular techniques can be utilized in the region of the descending thoracic aorta with subsequent resection of the involved aortic wall (19). Marulli’s group has reported on a series of 9 patients treated by placement of an endograft prior to resection with no mortality (20). These authors note that placement of an endograft allows a more safe performance of a subadventitial dissection in order to resect the tumor. Furthermore, two patients in this series had a one-stage procedure which the authors hypothesized might save patients an unnecessary procedure if at most only adventitial invasion is found at the time of thoracotomy.
Open repair of resected aorta with an interposition graft without CPB is also an option. However, in the region of the aortic arch or proximal descending aorta standard CPB with circulatory arrest if necessary may be utilized. Overall, in Marulli et al. series of 35 patients a multivariate analysis demonstrated a better prognosis for patients with involvement of the descending thoracic aorta than those with involvement of the aortic arch and/or supraortic vessels thought secondary to the increased surgical complexity of the latter group (18). Even in this series which included 11 patients with aortic arch and great vessel resections the overall mortality rate was 2.9% and morbidity rate was 37.1% of which intraoperative or postoperative bleeding was the most common problem.
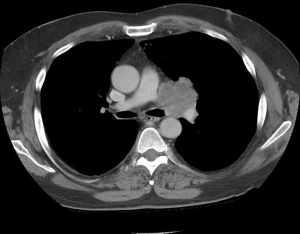
Resection of T4 lung cancers involving the pulmonary arterial trunk as noted above has been reported less frequently. Several small series have reported the use of CPB to resect and reconstruct a proximal pulmonary artery involved with the primary tumor (Figure 3). A total of 11 patients in these series underwent resection of the left main pulmonary artery with either patch reconstruction using polytetrafluoroethylene (PTFE) or pericardium while 3 of these cases reported employed homograft reconstruction of the main pulmonary artery trunk (9,16,21-23). Another 8 cases which were reported neither the sidedness nor technique of reconstruction were stated (9,24). It is noteworthy that in some of these cases nearby structures such as the aorta or left atrium were also involved which mandated the use of CPB for complete resection. It is hypothesized that the preponderance of left sided tumors in these series may be due to the anatomical fact that tumors involving the proximal right main pulmonary artery are not likely resectable from other mediastinal structures.
Tumors invading the SVC are less likely to require CPB for successful resection. Certainly noncircumferential or limited involvement of the SVC can be managed by various techniques such as partial occlusion, shunting or even complete occlusion. In the latter situation which also is necessary for more extensive involvement of the SVC the salient points of surgical conduct include maintenance of arterial pressure (mean of 100 mmHg) and anticoagulation (25). Some have also encouraged limiting the occlusion time to less than 60 minutes (26). The concern is that of cerebral hypoperfusion due to a sudden increased central venous pressure while potential decreased arterial perfusion pressure due to decrease cardiac venous return. This situation can be counteracted by use of vasoactive agents and IV fluid loading prior to clamping.
Preoperative imaging of the upper extremity venous vasculature and caval echocardiography is essential for assurance of resectability and long term patency. Utilizing either bovine or autologous pericardium for partial caval replacement or 18–20 mm PTFE for complete replacement allows successful reconstruction. A technique of “extra-anatomic” replacement of the SVC has also been reported (27). In these latter situations of complete replacement of the SVC with PTFE extended oral or subcutaneous anticoagulation for 6 months is recommended to prevent thrombosis of the graft.
All the above techniques for replacement of the SVC do not necessarily require CPB. In the rare instance of tumor involvement of the caval-atrial junction and/or the right atrium CPB may be necessary for complete reconstruction (12,28). Although the only reports of involvement of the inferior vena cava are for neoplasms other than lung cancer CPB was necessary for resection and reconstruction of this structure also (4,21).
LIMA grafts
The routine use of LIMA grafts in CABG surgery presents difficulty in left upper lobectomies as the graft is usually quite adhered to this lobe. In these situations preparation for possible revascularization may be prudent. Two studies from large academic centers have demonstrated that resection is possible without the use of CPB. Wei et al. in a series of 27 patients undergoing left upper lobectomy with a patent LIMA graft resection was accomplished with preservation of the graft and no perioperative myocardial infarction and 0% 30- and 90-day mortality (29). Complete dissection of the LIMA graft from the lobe was performed in 25 patients but thoracotomy was required. In a series of 290 patients undergoing left upper lobectomy by VATS of which 14 had a previous LIMA graft Shah et al. reported no mortality, similar perioperative morbidity (35.7% vs. 29.4% control, P=0.61) and similar 5-year survival (50% vs. 63% control, P=0.23) and overall recurrence rate (27% vs. 15% control, P=0.27) (30). In the latter study the technique of routinely leaving a protective small wedge of lung tissue attached to the LIMA graft was employed.
At our main institution which is a free standing cancer institute CPB is not available. We have, however, routinely performed VATS left upper lobectomies in patients with a patent LIMA graft without the use of CPB support or backup. All patients have a formal cardiology consultation including recommended testing. A CT angiogram and the CABG operative note are reviewed. We employ careful selection and various accepted techniques including leaving a small margin of benign lung tissue attached to the LIMA graft. Our results in a series of 25 patients demonstrate a similar incidence of short term morbidity and mortality in patients undergoing a VATS left upper lobectomy after having had a prior CABG with a LIMA graft in place when compared to a simultaneous cohort of patients undergoing lobectomy without a prior CABG (unpublished data). In particular there was no statistical increase in cardiac events including atrial arrhythmias, ventricular arrhythmias, myocardial infarction or mortality.
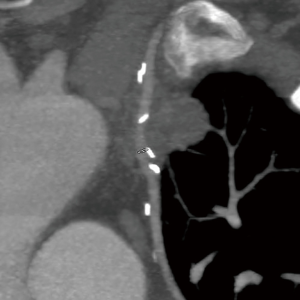
In one other case CT angiography demonstrated involvement of the LIMA graft itself by tumor creating a “pseudo” T4 situation (Figure 4). Although preoperative evaluation including catheterization revealed that sacrifice of the LIMA graft would be well tolerated the resection was done with CPB standby without incident. A redo trans-sternal approach was used with exposure of the heart, LIMA graft and test occlusion of the graft with electrocardiographic and transesophageal echocardiographic monitoring. In these rare situations preoperative planning, approach and techniques for revascularization are crucial. If CPB is required it should managed in a controlled fashion.
Tracheal/carinal resection
One of the earliest reports of use of CPB during resection of a carinal tumor (adenoma) was described using a right thoracotomy approach (31). Carinal or tracheal resection in order to accomplish an en bloc resection of T4 lung cancers present concerns for maintenance of airway control. Several techniques are available for dealing with this issue including cross field intubation, intermittent apnea, high frequency jet ventilation (HFJV), CPB or ECMO (32). Only the latter two techniques allow extended interruption of ventilation and are particularly suited for tracheal resections trans-sternally. The use of CPB/ECMO frees the airway from endotracheal catheters and allows less cumbersome dissection and anastomosis of airway structures in more complex cases (33).
The common approaches include a 4th or 5th intercostal space right thoracotomy, a median sternotomy or a clam-shell approach through the 4th intercostal space. A bilateral thoracotomy technique has also been described for left sided lesions (34). Most carinal resections currently employ a cross field ventilation technique whether performed via sternotomy or thoracotomy. A landmark single institution report of 143 carinal resections in 135 patients noted that only 2 patients required CPB (one for pulmonary hemorrhage and one for associated pulmonary artery resection) (35). Recently, a publication by Dartevelle et al. described a single institution experience with 138 carinal resections of which only 4 of these cases required CPB (25). These authors nicely describe in detail the extent of resection possible and the various techniques for tracheobronchial reconstruction. Using their techniques they were able to achieve a 96.4% R0 resection rate.
Most resections are either right or left carinal sleeve pneumonectomies although sleeve right upper lobectomies can also be performed. Mortality rates in these complex surgeries range from 2–20% with 5-year survival rates of 19–47% (25,32). In the Mitchell series a subset of 60 patients undergoing carinal resection for lung cancer the 5-year survival for N0, N1, and N2/N3 disease was 51%, 32% and 12%, respectively (36). In the more recently published Dartevelle series the mortality rate was increased from 6.7% to 13% with the use of neoadjuvant chemotherapy while N0–N1 vs. N2–N3 nodal status decreased the 5-year survival from 47% to 24.4%. Finally, anastomotic morbidity was strongly associated with increased early postoperative mortality (OR 7.07; P=0.04) (35).
In summary, carinal resections for NSCLC can be performed safely. The use of CPB for this procedure is occasionally necessary in extenuating circumstances but has not been routinely employed. Attention to detail in performing tension free anastomoses is crucial for limiting operative morbidity and mortality.
Combined lung cancer resection and cardiac surgery
There have been many reports of lung resections during primary open heart procedures. The earliest report appeared in 1978 in which 3 patients underwent lung cancer resection during a CABG procedure (37). As noted in the abstract these early experiences with the use of CPB during lung cancer resection may have been the forerunner for the use of CPB in surgical resection of advanced T4 stage lesions. Resections in these combined surgeries have included wedge resection, lobectomy and pneumonectomy (38). Most often these situations occur as the patient either has an undiagnosed but resectable nodule or the severity of the cardiac abnormality would result in a predicted inordinate risk of resection without its amelioration. Here the principle issue is the whether the risk of performing staged procedures with the incurred delay in resection is less or greater than a combined operation. Many other factors such as patient preference, surgeon preference and institutional experience come into play. No clear distinction between these two choices has been reported. A recent review article of 15 published papers noted no significant difference between combined and staged procedures in operative mortality (0–20.8% vs. 0–10%, respectively) and 5-year survival (34.9–85% vs. 53%, respectively) but possibly a higher reoperation rate for bleeding (0–11% vs. 0%, respectively) (39).
Routinely, these combined procedures are performed through a sternotomy making right sided and upper lobe lesions more approachable. Left lower nodules and posteriorly located nodules are more difficult to expose. A thoracotomy incision can be made in addition but postoperative pain may be problematic. Most authors recommend lung resection prior to heparinization as major intraparenchymal bleeding has been reported to occur. However, the more recent advent of off-pump coronary artery bypass and interventional techniques has offered alternative approaches for correction of cardiac disease. The same authors above noted in review of 5 published papers that the operative mortality was 0–6.6%, 5-year survival was 13–68% and reoperation for bleeding was 4% in patients undergoing off pump CABG and lung resection (39). These data compare favorably to on pump CABG but with less concern of the negative effects of CPB during lung resection. Nowadays, therefore the occasion of combined lung resection and on pump cardiac operations has diminished to the point of almost insignificance.
Injuries during lung resection
Unfortunately, rarely injuries to major vascular structures can occur during a planned lung resection. Often times, the initial approach is a minimally invasive one and immediate control by various occlusive techniques is paramount to a successful outcome. If major structures such as a pulmonary artery, superior or inferior vena cava, left or right atrium are injured emergent establishment of CPB may be life-saving. This most often can be accomplished by femoral venous and arterial access if the patient can be placed in a thoracoabdominal position but otherwise will require a thoracotomy with a transsternal extension in order to obtain adequate exposure to necessary cardiac structures. At least one study has shown that in the emergent setting overall survival is significantly reduced (10).
As a corollary topic, mediastinoscopy for staging of lung cancer has been associated with difficult to manage hemorrhage. Injury to the azygos vein, right main pulmonary artery or innominate artery can occur resulting in massive hemorrhage. Again, immediate control by occlusive techniques is imperative. Emergent right thoracotomy may be necessary for control of azygos venous hemorrhage and stenting of the innominate artery can be employed to control hemorrhage. The most difficult structure to repair is injury to the right main pulmonary artery which will usually require CPB for management. The anatomic location of this structure within the mediastinum makes proximal surgical control problematic without CPB whether through a sternotomy or right thoracotomy (personal experience).
Summary
Resection of locally advanced T4 NSCLC is being performed with increased frequency. Selective utilization of CPB in the elective setting has been shown to be safe and effective. Achieving an R0 resection is paramount for optimal long-term survival. Most authors agree that lymph node status in these advanced tumors is as important for survival as in any NSCLC case and therefore should be part of the preoperative assessment. N2 or N3 nodal disease corresponding to stage IIIa and IIIb status respectively are not likely to result in satisfactory rates of long-term survival and require case by case selection. The use of neoadjuvant and/or adjuvant therapy may improve long-term survival. However, at least in the former situation there may be increased operative morbidity and mortality. Lung sparing techniques with the avoidance of pneumonectomy if possible appears to be associated with improved outcomes. Although use of CPB in an emergent situation of bleeding may be lifesaving it is most effective when employed in an elective well planned and executed manner. Finally, in the appropriate situation use of ECMO as opposed to CPB may be a preferred modality.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Xiangya Medicine for the series “Extended Pulmonary Resections for T4 Non-Small-Cell-Lung-Cancer”. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2018.05.02). The series “Extended Pulmonary Resections for T4 Non-Small-Cell-Lung-Cancer” was commissioned by the editorial office without any funding or sponsorship. SY served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sellke FW, Del Nido PJ, Swanson SJ. Sabiston & Spencer surgery of the chest. 9th edition. Philadelphia, PA: Elsevier, 2016;2:1849-57.
- Silverman NA. Primary cardiac tumors. Ann Surg 1980;191:127-38. [Crossref] [PubMed]
- Skhvatsabaja LV. Secondary malignant lesions of the heart and pericardium in neoplastic disease. Oncology 1986;43:103-6. [Crossref] [PubMed]
- Vaporciyan AA, Rice D, Correa AM, et al. Resection of advanced thoracic malignancies requiring cardiopulmonary bypass. Eur J Cardiothorac Surg 2002;22:47-52. [Crossref] [PubMed]
- Delarue NC, Eschapasse H. Lung cancer. Philadelphia: W.B. Saunders, 1985;315:88-99.
- Tsuchiya R. Rinsho Kyobu Geka 1987;7:440-3. [Prognosis of the patients with lung cancer treated by combined left atrium or great vessel resection]. [PubMed]
- Ricci C, Rendina EA, Venuta F, et al. Reconstruction of the pulmonary artery in patients with lung cancer. Ann Thorac Surg 1994;57:627-32; discussion 632-3. [Crossref] [PubMed]
- Rami-Porta R, Bolejack V, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:990-1003.
- Langer NB, Mercier O, Fabre D, et al. Outcomes After Resection of T4 Non-Small Cell Lung Cancer Using Cardiopulmonary Bypass. Ann Thorac Surg 2016;102:902-10. [Crossref] [PubMed]
- Muralidaran A, Detterbeck FC, Boffa DJ, et al. Long-term survival after lung resection for non-small cell lung cancer with circulatory bypass: a systematic review. J Thorac Cardiovasc Surg 2011;142:1137-42. [Crossref] [PubMed]
- Yang HX, Hou X, Lin P, et al. Survival and risk factors of surgically treated mediastinal invasion T4 non-small cell lung cancer. Ann Thorac Surg 2009;88:372-8. [Crossref] [PubMed]
- Park BJ, Bacchetta M, Bains MS, et al. Surgical management of thoracic malignancies invading the heart or great vessels. Ann Thorac Surg 2004;78:1024-30. [Crossref] [PubMed]
- Kuehnl A, Lindner M, Hornung HM, et al. Atrial resection for lung cancer: morbidity, mortality, and long-term follow-up. World J Surg 2010;34:2233-9. [Crossref] [PubMed]
- Lang G, Taghavi S, Aigner C, et al. Extracorporeal membrane oxygenation support for resection of locally advanced thoracic tumors. Ann Thorac Surg 2011;92:264-70. [Crossref] [PubMed]
- Marseu K, Minkovich L, Zubrinic M, et al. Anesthetic Considerations for Pneumonectomy With Left Atrial Resection on Cardiopulmonary Bypass in a Patient With Lung Cancer: A Case Report. A A Case Rep 2017;8:61-3. [Crossref] [PubMed]
- Wiebe K, Baraki H, Macchiarini P, et al. Extended pulmonary resections of advanced thoracic malignancies with support of cardiopulmonary bypass. Eur J Cardiothorac Surg 2006;29:571-7. [Crossref] [PubMed]
- de Perrot M, Fadel E, Mussot S, et al. Resection of locally advanced (T4) non-small cell lung cancer with cardiopulmonary bypass. Ann Thorac Surg 2005;79:1691-6; discussion 1697.
- Marulli G, Rendina EA, Klepetko W, et al. Surgery for T4 lung cancer invading the thoracic aorta: Do we push the limits? J Surg Oncol 2017;116:1141-9. [Crossref] [PubMed]
- Collaud S, Waddell TK, Yasufuku K, et al. Thoracic aortic endografting facilitates the resection of tumors infiltrating the aorta. J Thorac Cardiovasc Surg 2014;147:1178-82; discussion 1182. [Crossref] [PubMed]
- Marulli G, Rea F, Zampieri D, et al. Safe resection of the aortic wall infiltrated by lung cancer after placement of an endoluminal prosthesis. Ann Thorac Surg 2015;99:1768-73. [Crossref] [PubMed]
- Byrne JG, Leacche M, Agnihotri AK, et al. The use of cardiopulmonary bypass during resection of locally advanced thoracic malignancies: a 10-year two-center experience. Chest 2004;125:1581-6. [Crossref] [PubMed]
- Mei J, Pu Q, Zhu Y, et al. Reconstruction of the pulmonary trunk via cardiopulmonary bypass in extended resection of locally advanced lung malignancies. J Surg Oncol 2012;106:311-5. [Crossref] [PubMed]
- Venuta F, Ciccone AM, Anile M, et al. Reconstruction of the pulmonary artery for lung cancer: long-term results. J Thorac Cardiovasc Surg 2009;138:1185-91. [Crossref] [PubMed]
- Hasegawa S, Bando T, Isowa N, et al. The use of cardiopulmonary bypass during extended resection of non-small cell lung cancer. Interact Cardiovasc Thorac Surg. 2003;2:676-9. [Crossref] [PubMed]
- Dartevelle PG, Mitilian D, Fadel E. Extended surgery for T4 lung cancer: a 30 years' experience. Gen Thorac Cardiovasc Surg 2017;65:321-8. [Crossref] [PubMed]
- Tanaka Y, Hokka D, Ogawa H, et al. Surgery for malignant lesions of the chest which extensively involved the mediastinum, lung, and heart. Gen Thorac Cardiovasc Surg 2017;65:365-73. [Crossref] [PubMed]
- Lanuti M, De Delva PE, Gaissert HA, et al. Review of superior vena cava resection in the management of benign disease and pulmonary or mediastinal malignancies. Ann Thorac Surg 2009;88:392-7. [Crossref] [PubMed]
- Spaggiari L, Leo F, Veronesi G, et al. Superior vena cava resection for lung and mediastinal malignancies: a single-center experience with 70 cases. Ann Thorac Surg 2007;83:223-9; discussion 229-30. [Crossref] [PubMed]
- Wei B, Broussard B, Bryant A, et al. Left upper lobectomy after coronary artery bypass grafting. J Thorac Cardiovasc Surg 2015;150:531-5. [Crossref] [PubMed]
- Shah AA, Worni M, Onaitis MW, et al. Thoracoscopic left upper lobectomy in patients with internal mammary artery coronary bypass grafts. Ann Thorac Surg 2014;98:1207-12. [Crossref] [PubMed]
- Woods FM, Neptune WB, Palatchi A. Resection of the carina and main-stem bronchi with the use of extracorporeal circulation. N Engl J Med 1961;264:492-4. [Crossref] [PubMed]
- Weder W, Inci I. Carinal resection and sleeve pneumonectomy. Thorac Surg Clin 2014;24:77-83. [Crossref] [PubMed]
- Lei J, Su K, Li XF, et al. ECMO-assisted carinal resection and reconstruction after left pneumonectomy. J Cardiothorac Surg 2010;5:89. [Crossref] [PubMed]
- Shin S, Park JS, Shim YM, et al. Carinal resection and reconstruction in thoracic malignancies. J Surg Oncol 2014;110:239-44. [Crossref] [PubMed]
- Mitchell JD, Mathisen DJ, Wright CD, et al. Clinical experience with carinal resection. J Thorac Cardiovasc Surg 1999;117:39-52; discussion 52-3. [Crossref] [PubMed]
- Mitchell JD, Mathisen DJ, Wright CD, et al. Resection for bronchogenic carcinoma involving the carina: long-term results and effect of nodal status on outcome. J Thorac Cardiovasc 2001;121:465-71. [Crossref] [PubMed]
- Dalton ML, Parker TM, Mistrot JJ, et al. Concomitant coronary artery bypass and major noncardiac surgery. J Thorac Cardiovasc Surg 1978;75:621-4. [PubMed]
- Yang Y, Xiao F, Wang J, et al. Simultaneous surgery in patients with both cardiac and noncardiac diseases. Patient Prefer Adherence 2016;10:1251-8. [Crossref] [PubMed]
- Tourmousoglou CE, Apostolakis E, Dougenis D. Simultaneous occurrence of coronary artery disease and lung cancer: what is the best surgical treatment strategy? Interact Cardiovasc Thorac Surg 2014;19:673-81. [Crossref] [PubMed]
Cite this article as: Picone AL, Yendamuri S. Use of cardiopulmonary bypass in lung cancer surgery: focus on extended pulmonary resections for T4 non-small cell lung cancer. J Xiangya Med 2018;3:24.


