Bronchial carcinoid, unusual manifestation: a case report
Introduction
Carcinoid tumors are neuroendocrine tumors that originate in the digestive tract, lungs, or rare primary sites, such as kidneys or ovaries. The term carcinoid usually implies a well-differentiated histology and is rarely used to describe high-grade or poorly differentiated neuroendocrine cancers. Carcinoid syndrome is the term applied to a constellation of symptoms that are associated with elevations in serum serotonin (5-HT) or its metabolite urinary 5-hydroxyindoleacetic acid (5-HIAA), or other various hormones that are secreted by some carcinoid tumors (1). Two of the most common manifestations are flushing and diarrhea. The manifestations can be provoked by some stressful situations and may last from several hours for up to 2 days (1,2). Carcinoid crisis is life threatening form of carcinoid syndrome that results from the release of an overwhelming amount of 5-HIAA from tumor and its manifestation including hemodynamic instability, acute right heart failure and/or shock (3). Typical bronchial carcinoids tend to grow slowly, near the center of the lungs, and may remain asymptomatic for a long time and may be reason of absence of lung perfusion even when atelectasis absents (4).
Case presentation
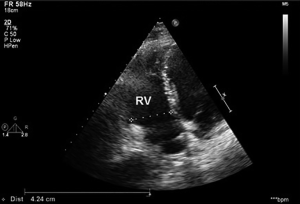
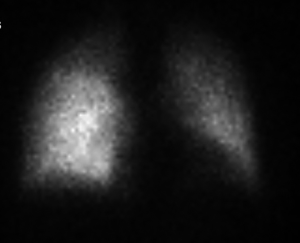
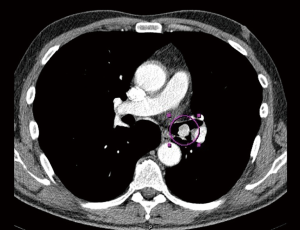
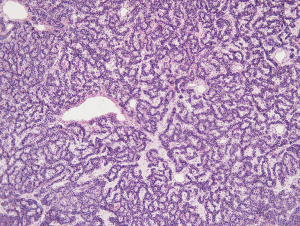
A 55-year-old Caucasian male, non-smoker, with no family history of cardiovascular diseases or malignancy, not on any outpatient medications, was sent to Department of Cardiology University Hospital in Martin with acute onset chest pain. He was diagnosed as an acute ST segment elevation posterolateral myocardial infarction (MI), with duration of ischemia 6 hours. He underwent successful PCI of circumflex artery with a intracoronary stent placement. Upon the admission, transthoracic echocardiography (TTE) showed non-dilated left ventricle with an ejection fraction of 45%, no aortic root dilation, no mitral or aortic valve regurgitation or stenosis, dilated right ventricle with end-diastolic diameter of 40 mm and no tricuspid regurgitation, no pulmonary hypertension and no pericardial effusion. Dilated right ventricle was believed to be a result of a posterolateral MI. Following day, the patient developed acute dyspnea without chest pain. He was tachycardic (100 bpm) and hypotensive (90/60 mmHg). He had bilaterally decreased breath sounds, jugular venous distention and positive hepatojugular reflux sign. Sinus tachycardia was found on ECG and recurrent MI was ruled out. Emergent TTE revealed progression of right ventricle dilation to 42.4 mm and new onset of pulmonary hypertension, with estimated pulmonary artery pressure of 54 mmHg, right ventricle systolic function was significantly reduced (Figure 1). Because of suspicion of pulmonary embolism, the patient was started with intravenous unfractionated heparin. As he was only 24 hours out of selective coronography and his creatinine was elevated, a lung perfusion scintigraphy was felt to be safer than CT angiopulmography. Lung perfusion scintigraphy revealed an absent perfusion of the entire left lung (Figure 2), while chest X-ray showed no parenchymal abnormality. Clinical presentation and absent perfusion of the entire left lung on perfusion scintigraphy and high level of D-dimer pointed towards the diagnosis of acute pulmonary embolism with high risk. At the same time, the patient was at high risk of bleeding if given thromboembolytic therapy and prior to considered transcathetral embolectomy, he did ultimately undergo CT angiopulmography, which ruled out pulmonary embolism, but discovered a mass at the left central bronchus (Figure 3). The patient was hemodynamic stabilized with symptomatic therapy alone and dyspnea resolved during next hours, as well. Subsequently, the patient underwent bronchoscopy that confirmed a polypoid mass partially obstructing left central bronchus, which appeared macroscopically as typical carcinoid. High levels of 5-HIAA supported the diagnosis. The definite diagnosis of typical bronchial carcinoid was confirmed by histology (Figure 4). The patient underwent octreoscan, that ruled out local or distant metastases. As a part of the pre-operative work up, repeated lung perfusion scintigraphy showed ongoing absence of left lung perfusion, patient was asymptomatic at that time. Six months after MI the patient underwent lung sparing resection without complications. With respect to the complete tumor resection and absence of metastases, according to oncologist and endocrinologist, he didn’t require any additional therapy. Three months after resection of tumor, a follow-up perfusion lung scintigraphy revealed restored perfusion of left lung (Figure 5), TTE demonstrated regression of right ventricle dilation and pulmonary hypertension and no tricuspid regurgitation. Presently, 5 years after lung sparing resection, the patient is without disease recurrence and according to result from TTE without development of chronic carcinoid heart disease (tricuspid valve insufficiency).
Discussion
Carcinoid tumors are neuroendocrine tumors, and the majority of them arise within the gastrointestinal tract (58%), respiratory system is further most common site of origin (27%) and the remaining part (15%) arises from other or unknown locations. Carcinoid tumors comprise 0.5% to 5% of all lung tumours and they are malignant tumors with the potential to metastasize. Typical bronchial carcinoids comprise about 90% of all pulmonary carcinoids and show a high degree of differentiation (5). Presenting symptoms of typical bronchial carcinoids include cough, recurrent pulmonary infection, hemoptysis, dyspnea, wheezing or fever. Some patients may be asymptomatic and their tumors are discovered incidentally. Carcinoid syndrome, which is characterized by excessive 5-HT release from tumors leading to hot, red flushing of the face, severe and debilitating diarrhea and asthma attacks (5,6). Gastrointestinal carcinoids secrete 5-HT (or other tumor-secreted substances) into the portal circulation, which is immediately metabolized in the liver and carcinoid syndrome occurs only in patients with liver metastases, because a large amount of 5-HT are not completely metabolized by hepatic and enter the systemic circulation, causing carcinoid symptoms. On the other hand, carcinoid syndrome is rarely associated with bronchial carcinoids, because extensive liver metastases are not present and 5-HT are completely metabolized by pulmonary cells (7). Diagnosis of typical bronchial carcinoids is complicated not only by late and non-specific symptoms but also due to technical diagnostics limits. Classical chest X-ray does not necessarily provide us with clinical significant information especially when the tumor is located centrally. Final diagnosis of bronchial carcinoids is based on bronchoscopic biopsy, but evaluation often initially involves chest CT, which reveals tumour (6), as we demonstrated in our patient. Pulmonary perfusion disorders visualized by radionuclide perfusion lung scanning associated with bronchus occlusion by tumor mass without atelectasis has been described by some authors (4,8,9). The cause of absent pulmonary perfusion even without atelectasis, which is localized on the same side as carcinoid can be explained by several pathological processes. Probably the main role plays vasoconstriction in pulmonary circulation caused by bronchial carcinoid (10) that induced hypoxaemia and hypoxaemia increases vasoconstriction effect of 5-HT in pulmonary circulation, which is released from carcinoid. The typical bronchial carcinoids are slow growing tumours and therefore it is assumed that carcinoid enhanced hypoxaemia is long lasting (11). After the tumor is removed, pulmonary perfusion returns to normal depending on the duration of hypoxaemia effect (9), as we demonstrated in our patient. Bronchial carcinoids are slow-growing tumors with 5-HT secretion directly into the systematic circulation and chronic cardiac manifestation usually develops in the late phase when the changes are already irreversible (12). Therefore, once carcinoid has been diagnosed, the patient should undergo an echocardiogram to rule out tricuspid valve insufficiency, and if it is present, these patients need to be referred to the cardiologist for definitive management (13). Carcinoid crisis is life threatening form of carcinoid syndrome that results from the release of an overwhelming amount of 5-HT from tumor and its manifestation including hemodynamic instability, acute right heart failure and/or shock and may be triggered by tumor manipulation (biopsy, surgery), anesthesia, sedatives, catecholamines or by other stressful situations (1,3,14,15). For our presented patient, acute MI might be a stressful situation. More than 90% of patients with carcinoid syndrome have metastatic disease, typically involving the liver. Rare exception are bronchial and ovarian carcinoids, which can rarely release hormones directly into the system circulation, thereby producing symptoms without metastases. When it does occur carcinoid syndrome and/or crisis associated with bronchial carcinoids is often atypical, episodes of flushing and/or diaphoresis may be accompanied by other symptoms, such tremor, periorbital edema, lacrimation, salivation and edema (16). Patients with carcinoid crisis can present with severe hemodynamic instability due to severe bronchoconstriction, cyanosis, acute right heart failure and hypotension (1,3,15). The main pillar of treatment for carcinoid syndrome and crisis is the use of somatostatin analogues, such as octreotide and lanreotide. Approximately 80% of typical carcinoids express somatostatin receptors in the carcinoid cell surface (7). In the case of hypotension, calcium products and catecholamine should be avoided as they worsen release of 5-HT from the tumor. The severe hypotension can be treated either by using vasopressin or inhibitors of phosphodiesterase (17). Lung sparing resection as a primary therapy seems to be adequate for most bronchial carcinoids, with complete resection yielding excellent local control and long-term survival. Surgery has been reported to provide a 5- and 10-year survival rate of >90% for typical bronchial carcinoid. Adjuvant radiotherapy or chemoradiation have not been well studied, but should be considered for atypical bronchial carcinoids with concerning pathologic features until data is available indicating otherwise (5). The prognosis depends on the histology classification (typical and/or atypical carcinoids) and presence or absence of metastases and lymph node involvement. Typical bronchial carcinoids and atypical bronchial carcinoids have a recurrence rate of approximately 3–5% and 25%, respectively. Thus, adjuvant therapy should be considered in completely resected typical and atypical carcinoids with mediastinal lymph node involvement and/or adverse pathologic features (5,18).
In conclusion, typical bronchial carcinoids are a relatively rare subset of pulmonary tumors that often are asymptomatic and their first manifestation may be atypical carcinoid crisis, provoked by stressful situation (for example acute MI) that may mimic acute pulmonary embolism.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2019.09.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Aluri V, Dillon JS. Biochemical Testing in Neuroendocrine Tumors. Endocrinol Metab Clin North Am 2017;46:669-77. [Crossref] [PubMed]
- Palaniswamy C, Frishman WH, Aronow WS. Carcinoid heart disease. Cardiol Rev 2012;20:167-76. [Crossref] [PubMed]
- Majeed F, Porter TR, Tarantolo S, et al. Carcinoid crisis and reversible right ventricular dysfunction after embolization in untreated carcinoid syndrome. Eur J Echocardiogr 2007;8:386-9. [Crossref] [PubMed]
- Cei M, Mumoli N, Mariotti F, et al. The importance of clinical suspicion in diagnosing pulmonary embolism: a case of false-positive high probability radionuclide perfusion lung scan. Eur J Emerg Med 2004;11:234-6. [Crossref] [PubMed]
- Herde RF, Kokeny KE, Reddy CB, et al. Primary Pulmonary Carcinoid Tumor: A Long-term Single Institution Experience. Am J Clin Oncol 2018;41:24-9. [PubMed]
- Fink G, Krelbaum T, Yellin A, et al. Pulmonary carcinoid: presentation, diagnosis, and outcome in 142 cases in Israel and review of 640 cases from the literature. Chest 2001;119:1647-51. [Crossref] [PubMed]
- Rubin de Celis Ferrari AC, Glasberg J, Riechelmann RP. Carcinoid syndrome: update on the pathophysiology and treatment. Clinics (Sao Paulo) 2018;73:e490s. [PubMed]
- Caristo V, Mansberg R, Mansberg V, et al. A False Positive Lung Scan In A Middle Aged Man Due To Encasement Of Pulmonary Vasculature By Atypical Mediastinal Carinoid Tumour Demonstrated On SPECT/CT. J Nuclear Med 2007;3:10-3.
- Balan KK, Sonada L, Siraj QH. Ventilation-Perfusion Scanning before and after surgery in a patient with a carcinoid tumour. PJNM 2011;1:81-4.
- Jafri S, Sivasothy P, Wells F, et al. Clinical demonstration of efficiency and reversibility of hypoxic pulmonary vasoconstriction in a patient presenting with unilateral incomplete bronchial occlusion. Pulm Circ 2011;1:119-21. [Crossref] [PubMed]
- Egermayer P, Town GI, Peacock AJ. Role of serotonin in the pathogenesis of acute and chronic pulmonary hypertension. Thorax 1999;54:161-8. [Crossref] [PubMed]
- Bernheim AM, Connolly HM, Pellikka PA. Carcinoid heart disease in patients without hepatic metastases. Am J Cardiol 2007;99:292-4. [Crossref] [PubMed]
- Kundi H, Popma JJ, Cohen DJ, et al. Prevalence and Outcomes of Isolated Tricuspid Valve Surgery Among Medicare Beneficiaries. Am J Cardiol 2019;123:132-8. [Crossref] [PubMed]
- Kulke MH. Clinical presentation and management of carcinoid tumors. Hematol Oncol Clin North Am 2007;21:433-55. vii-viii. [Crossref] [PubMed]
- Janssen M, Salm EF, Breburda CS, et al. Carcinoid crisis during transesophageal echocardiography. Intensive Care Med 2000;26:254. [Crossref] [PubMed]
- Pasieka JL, Longman RS, Chambers AJ, et al. Cognitive impairment associated with carcinoid syndrome. Ann Surg 2014;259:355-9. [Crossref] [PubMed]
- Riechelmann RP, Pereira AA, Rego JF, et al. Refractory carcinoid syndrome: a review of treatment options. Ther Adv Med Oncol 2017;9:127-37. [Crossref] [PubMed]
- Zhong CX, Yao F, Zhao H, et al. Long-term outcomes of surgical treatment for pulmonary carcinoid tumors: 20 years' experience with 131 patients. Chin Med J (Engl) 2012;125:3022-6. [PubMed]
Cite this article as: Belicová M, Prídavková D, Jankovičová V, Balážová K, Mokáň M. Bronchial carcinoid, unusual manifestation: a case report. J Xiangya Med 2020;5:7.