
Association of diastolic blood pressure with history of stroke in community hypertensive patients: a cross-sectional study
Introduction
Stroke is the leading cause of death and disability worldwide, and the economic cost of treatment and care is huge. With more than 2 million new cases each year in China, stroke is associated with the highest disability-adjusted life year caused by any disease (1,2). In addition, stroke is a complex disease with many related risk factors. Among the many risk factors, hypertension is the most prevalent and treatable cause for stroke and other vascular events (3,4). Previous studies have shown that blood pressure (BP) control, especially systolic blood pressure (SBP) reduction, can significantly reduce the incidence of cardiovascular events; however, stroke events were not decreased (5). Another study including 0.5 million Chinese adults concluded that SBP was continuously related to major vascular disease, especially stroke (6). However, fewer studies directly assessed how diastolic blood pressure (DBP) might predict the risk of stroke. In 1999, Voko et al. found that the relationship between DBP and risk of stroke was J-shaped in treated hypertensives (7). Other authors summarized that the relation between BP reduction and cardiovascular risk may follow a J-shaped curve (8,9). Vidal-Petiot et al. reported a J-shaped association of DBP with cardiovascular events (except for stroke) in patients with coronary artery disease (CAD) treated for hypertension (10). Recently, however, a linear relationship has been assumed to challenge the J-shaped finding but this new relationship needs more data from large and randomized controlled trials to provide definitive answers (11,12).
In summary, there is still a lot of controversy about the relationship between DBP and risk of stroke, especially the question of how much DBP should be reduced to the most appropriate. Regarding that a limited number of researches studied the correlation between DBP and stroke events in hypertensives, not to mention studies based on the Chinese population, the present cross-sectional study aimed to explore the cross-sectional relationship between DBP and prior stroke in community hypertensive patients in China.
Methods
Study design and setting
This cross-sectional study was conducted at Liaobu, China, from January 2013 to December 2013. Researchers obtained demographic characteristics, performed physical examination, laboratory tests, as well as obtaining information on past medical history and medical prescriptions. Stroke cases were self-reported by patients. When participants attended the annual health examination, history of stroke was also recorded by trained interviewer.
Study participants
We included patients with at least 18 years old with diagnosed essential hypertension. We excluded patients that had missing data on blood pressure measurement and blood tests (Figure 1). A total of 8,130 participants were enrolled for data analysis. This study complied with the principles outlined in the Declaration of Helsinki. Institutional medical ethical committee from Guangdong Provincial People’s Hospital, China has approved this study. All participants have provided informed consent in written form.
Data collection and variables
Well-trained staff conducted a structured questionnaire to acquire information on demographic characteristics and social-economic factors (including age, sex, smoking, and drinking), medical history [diabetes mellitus (DM), CAD, and stroke], and use of antihypertensive drugs. Anthropometry and biomarkers including body mass index (BMI), SBP, DBP, heart rate, fasting blood glucose (FBG), estimated glomerular filtration rate (eGFR), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), triglyceride (TG) were assessed by laboratory analyses. Patients with SBP ≥140 mmHg, and/or DBP ≥90 mmHg, and/or use of antihypertensive medicine within 2 weeks were classified as hypertensive patients, with reference to 2010 Chinese guidelines for the management of hypertension (13). Diabetic patients were classified by diagnosis made by registered medical practitioner, the use of antidiabetic drugs within 2 weeks, and/or with FBG ≥7.0 mmol/L. Stroke cases were adjudicated by a neurologist in a hospital based on the self-reported history of stroke and cranial computed tomography or magnetic resonance imaging.
Statistical analysis
First, we categorized patients into five groups by their DBP levels by 10 mmHg increment, then tested the normality of data using Kolmogorov-Smirnov test. Baseline characteristics were presented as mean ± standard error for normally distributed continuous variables and numbers (percentages) for categorical variables, as appropriate. We detected subgroup differences by one-way ANOVA, Kruskal-Wallis H test and Chi-square test according to the normality of data distribution. Second, we performed logistic regression model to evaluate the associations of DBP with stroke. Restricted cubic spline was then performed to evaluate whether the non-linear relationship existed. For any non-linearity in association being detected, two-piecewise linear regression model was used to calculate the threshold values of DBP in predicting stroke. All analyses were performed with the statistical software package R 3.5.1 (https://cran.r-project.org/mirrors.html), statistical significance was detected by P values less than 0.05.
Results
Baseline characteristics of study participants
Table 1 showed the baseline characteristics of 8,130 participants. The average age was 64.01±12.40 years, 46.7% were men, and approximately 26.3% of them were smokers. There were 310 cases of stroke events. No statistically significant difference was found in smoking status and prevalence of stroke among different DBP groups. Age, sex, BMI, SBP, DBP, drink status, heart rate, eGFR, TC, TG, LDL-C, HDL-C, FBG, use of antihypertensive drugs, CAD and DM prevalence differed significantly by DBP values.
Table 1
| Characteristics | DBP groups | P value | ||||
|---|---|---|---|---|---|---|
| <60 (n=33) | ≥60, <70 (n=684) | ≥70, <80 (n=2,435) | ≥80, <90 (n=3,406) | ≥90 (n=1,572) | ||
| Age (years) | 74.667±9.433 | 68.098±13.638 | 64.451±13.837 | 62.628±13.110 | 56.260±13.183 | <0.001 |
| Sex (n, %) | <0.001 | |||||
| Male | 13 (39.394) | 284 (41.520) | 1,086 (44.600) | 1,550 (45.508) | 864 (54.962) | |
| Female | 20 (60.606) | 400 (58.480) | 1,349 (55.400) | 1,856 (54.492) | 708 (45.038) | |
| BMI (kg/m2) | 21.698±4.468 | 22.914±3.739 | 24.378±3.901 | 25.187±3.957 | 25.892±3.779 | <0.001 |
| SBP (mmHg) | 111.212±19.765 | 116.180±17.353 | 123.455±12.508 | 131.947±11.956 | 145.569±14.763 | <0.001 |
| DBP (mmHg) | 54.515±3.906 | 63.776±3.314 | 73.450±3.269 | 82.346±2.964 | 95.356±6.262 | <0.001 |
| Smoking (n, %) | 0.184 | |||||
| No | 24 (72.727) | 494 (72.222) | 1,798 (73.840) | 2,550 (74.868) | 1,128 (71.756) | |
| Yes | 9 (27.273) | 190 (27.778) | 637 (26.160) | 856 (25.132) | 444 (28.244) | |
| Drinking (n, %) | <0.001 | |||||
| No | 33 (100.000) | 624 (91.228) | 2,162 (88.789) | 2,983 (87.581) | 1,253 (79.707) | |
| Yes | 0 (0.000) | 60 (8.772) | 273 (11.211) | 423 (12.419) | 319 (20.293) | |
| Heart rate (beats/min) | 69.758±10.753 | 69.465±11.069 | 70.823±11.143 | 71.623±11.365 | 72.917±11.537 | <0.001 |
| eGFR (mL/min/1.73 m2) | 87.423±34.875 | 100.232±37.357 | 105.399±47.092 | 105.765±54.989 | 108.704±44.690 | 0.001 |
| TC (mg/dL) | 189.527±40.185 | 197.546±49.106 | 197.286±45.175 | 201.900±45.837 | 206.491±44.494 | <0.001 |
| TG (mg/dL) | 127.150±83.963 | 135.309±128.284 | 148.941±114.138 | 167.605±150.898 | 191.068±185.192 | <0.001 |
| LDL-C (mg/dL) | 90.351±27.397 | 97.805±29.354 | 96.871±27.908 | 98.492±28.578 | 100.173±28.794 | 0.004 |
| HDL-C (mg/dL) | 53.891±14.793 | 50.602±16.658 | 49.166±14.989 | 48.247±13.164 | 48.187±17.799 | <0.001 |
| FBG (mmol/L) | 4.771±1.250 | 5.131±1.715 | 5.250±1.831 | 5.125±1.524 | 5.062±1.466 | 0.003 |
| Antihypertensive drugs (n, %) | <0.001 | |||||
| No | 22 (66.667) | 438 (64.035) | 1,253 (51.458) | 1,387 (40.722) | 540 (34.351) | |
| Yes | 11 (33.333) | 246 (35.965) | 1,182 (48.542) | 2,019 (59.278) | 1,032 (65.649) | |
| CAD (n, %) | 0.002 | |||||
| No | 30 (90.909) | 653 (95.468) | 2,353 (96.632) | 3,296 (96.770) | 1,543 (98.155) | |
| Yes | 3 (9.091) | 31 (4.532) | 82 (3.368) | 110 (3.230) | 29 (1.845) | |
| DM (n, %) | <0.001 | |||||
| No | 29 (87.879) | 539 (78.801) | 1,885 (77.413) | 2,780 (81.621) | 1,323 (84.160) | |
| Yes | 4 (12.121) | 145 (21.199) | 550 (22.587) | 626 (18.379) | 249 (15.840) | |
| Stroke (n, %) | 3 (9.091) | 32 (4.678) | 99 (4.066) | 116 (3.406) | 60 (3.817) | 0.209 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; eGFR, estimated glomerular filtration rate; TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; FBG, fasting blood glucose; CAD, coronary artery disease; DM, diabetes mellitus.
Relationship between DBP and stroke
Results from logistic regression are summarized in Table 2, but not significant associations were found. We then presented results from minimally and fully adjusted models in Table 3. In the non-adjusted model, DBP showed did not associate with stroke (OR: 0.991, 95% CI, 0.980, 1.003). Fully adjusted models also demonstrated no significant associations. For sensitivity analysis, we treated DBP as a categorical variable, and did not find significant trend (P for trend was 0.605).
Table 2
| Covariates | OR | 95% CI | P value |
|---|---|---|---|
| Age | 1.033 | 1.024, 1.042 | <0.001 |
| Sex | |||
| Male | 1.0 | ||
| Female | 0.742 | 0.591, 0.932 | 0.010 |
| Smoking | |||
| No | 1.0 | ||
| Yes | 1.481 | 1.165, 1.883 | 0.001 |
| Drinking | |||
| No | 1.0 | ||
| Yes | 1.029 | 0.738, 1.434 | 0.866 |
| DM | |||
| No | 1.0 | ||
| Yes | 1.132 | 0.858, 1.495 | 0.381 |
| CAD | |||
| No | 1.0 | ||
| Yes | 2.895 | 1.884, 4.447 | <0.001 |
| Antihypertensive | |||
| Yes | 1.0 | ||
| No | 2.774 | 2.123, 3.624 | <0.001 |
| SBP | 1.006 | 0.999, 1.013 | 0.080 |
| DBP | 0.991 | 0.980, 1.003 | 0.138 |
| BMI | 0.996 | 0.967, 1.025 | 0.770 |
| Heart rate | 0.996 | 0.986, 1.007 | 0.489 |
| eGFR | 0.996 | 0.993, 0.999 | 0.004 |
| TC | 0.993 | 0.990, 0.996 | <0.001 |
| TG | 1.000 | 1.000, 1.001 | 0.431 |
| LDL-C | 0.992 | 0.987, 0.996 | <0.001 |
| FBG | 1.000 | 0.931, 1.074 | 0.999 |
OR, odds ratios; CI, confidence interval; DM, diabetes; CAD, coronary artery disease; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; eGFR, estimated glomerular filtration rate; TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; FBG, fasting blood glucose.
Table 3
| Exposure | Non-adjusted model (OR, 95% CI, P value) | Adjust I model (OR, 95% CI, P value) | Adjust II model (OR, 95% CI, P value) |
|---|---|---|---|
| DBP (per 10 mmHg change) | 0.991 (0.980, 1.003) 0.138 | 0.993 (0.978,1.009) 0.388 | 0.998 (0.982,1.014) 0.800 |
| DBP groups | |||
| <60 | 1.0 | 1.0 | 1.0 |
| ≥60, <70 | 0.491 (0.142, 1.694) 0.260 | 0.516(0.148,1.804) 0.300 | 0.510(0.139,1.870) 0.310 |
| ≥70, <80 | 0.424 (0.127, 1.412) 0.162 | 0.468 (0.138,1.586) 0.223 | 0.404 (0.114,1.437) 0.162 |
| ≥80, <90 | 0.353 (0.106, 1.172) 0.089 | 0.399 (0.117,1.360) 0.142 | 0.370 (0.104,1.322) 0.126 |
| ≥90 | 0.397 (0.118, 1.337) 0.136 | 0.491 (0.137,1.759) 0.274 | 0.489 (0.131,1.826) 0.287 |
| P for trend | 0.130 | 0.426 | 0.605 |
OR, odds ratios; CI, confidence interval; DBP, diastolic blood pressure. Adjust I model: adjust for age, sex, systolic blood pressure and body mass index. Adjust II model: adjust for age, sex, body mass index, systolic blood pressure, smoking, drinking, estimated glomerular filtration rate, heart rate, total cholesterol, triglyceride, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, fasting blood glucose, diabetes mellitus, and antihypertensive drugs.
The analysis of the non-linear relationship
In Figure 2, we found that non-linear relationship between DBP and stroke in fully adjusted model. According to the two-piecewise linear regression model, the inflection point of DBP was 80 mmHg. A negative relationship between DBP and stroke was only significant for patients with DBP <80 mmHg (OR: 0.969, 95% CI, 0.948, 0.991) (Table 4).
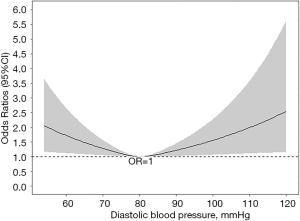
Table 4
| Inflection point of DBP (per 10 mmHg change) | OR | 95% CI | P value |
|---|---|---|---|
| <80 | 0.969 | 0.948, 0.991 | 0.005 |
| ≥80 | 1.008 | 0.991, 1.027 | 0.353 |
Adjust for age, sex, body mass index, systolic blood pressure, smoking, drinking, estimated glomerular filtration rate, heart rate, total cholesterol, triglyceride, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, fasting blood glucose, diabetes mellitus, and antihypertensive drugs. OR, odds ratios; CI, confidence interval; DBP, diastolic blood pressure.
Discussion
We studied the cross-sectional relationship between DBP and prior stroke among 8,130 hypertensive patients in China. A total of 310 stroke events occurred during the whole year. Neither the univariable logistic regression analysis nor the multivariate adjustment models found a statistically significant relationship between DBP and stroke. However, a non-linear relationship between DBP and stroke was observed using restricted cubic spline. The two-piecewise linear regression model showed a cut-off point of 80 and a negative relationship between DBP and stroke was discovered on the left side of the inflection point.
It has been widely debated whether a J-curve phenomenon reflecting an adverse relationship between excessive BP reduction and cardiovascular risk exists in prognosis prediction, especially for stroke. The SPRINT (Systolic Blood Pressure Intervention Trial) concluded that achieving SBP <120 mmHg resulted in fewer major cardiovascular events and all-cause death compared with <140 mmHg, but the number of strokes was not reduced (HR, 0.89; 95% CI, 0.63–1.25; P=0.50) (5). SPRINT results showed benefits and safety of BP reduction and have profoundly affected the newest US guidelines on management of hypertension in adults, adopting lower BP criteria for the definition of hypertension from the previous 140/90 to 130/80 mmHg (14). Although the latest 2018 ESC/ESH Guidelines for the management of arterial hypertension still maintained 140/90 mmHg as diagnostic criteria, it was recommended that all patients with hypertension should be reduced DBP down to below 80 mmHg, independent of the level of risk and comorbidity (15). It also emphasized that high normal BP (130–139/85–89 mmHg) should consider drug treatment when cardiovascular risk is very high (15). Besides, the Rotterdam observational study showed that higher rates of stroke happened in elderly hypertensive participants who were treated to DBP <60 mmHg compared with DBP 65–74 mmHg (7). These findings supported the idea of the J-curve phenomenon.
However, the HOT (Hypertension Optimal Treatment) trial (16) found that advantages from tight BP control were not so obvious. No direct effect on stroke risk of DBP reduction to ≤90, ≤85, ≤80 or 70 mmHg in the overall patients or the subgroup participants with DM was discovered. Similarly, Vidal-Petiot et al. (10) also revealed that among patients with stable CAD, DBP <60 mmHg or at 60–69 mmHg had no significant effect on stroke risk comparing to DBP at 70–79 mmHg. Nevertheless, in the ACCORD (Action to Control Cardiovascular Risk in Type 2 Diabetes) trial, there were fewer stroke events in the intensive BP control group, but benefits for other endpoints were not seen. Among these patients with DM, DBP 64.4 mmHg of intensive therapy resulted in a significant reduction in all strokes (HR, 0.59; 95% CI, 0.39–0.89; P=0.01) and non-fatal strokes (HR, 0.63; 95% CI, 0.41–0.96; P=0.03) compared with 70.5 mmHg of standard therapy (17). In accordance with the ACCORD study, the SHEP (Systolic Hypertension in the Elderly Program) trial displayed that patients with a DBP of less than 70 mmHg in the active BP control group had fewer strokes, and in patients older than 60 years, intensive treatment of DBP down to 68 mmHg had a lower risk of stroke than the placebo group with DBP 72 mmHg (relative risk was 0.64; 95% CI, 0.50–0.82; P=0.0003) (18). Another study named Hypertension in the Very Elderly Trial (19), suggesting the cause-and-effect relationship between low DBP and adverse cerebrovascular outcomes, indicated that DBP <80 mmHg was associated with a significant decrease in fatal stroke events. This result was contrary to our present study given that we found stroke risk is higher when DBP <80 mmHg. This disagreement could be attributed to the difference of specific study population and clinical variables that were considered, collected and adjusted.
In addition, several studies have questioned the ability of DBP to be used independently for risk prediction. A nationwide population-based study from Korea found that both SBP and DBP increased cardiovascular risk in a J-shaped manner which could extend down to 90/40 mmHg, but the association of DBP independent of SBP was variable. These authors raised that the difference between DBP <80 mmHg and 80–89 mmHg for estimate cardiovascular risk disappeared after adjustment (20). Sobieraj et al. (21) used the SPRINT data to investigate how low DBP influenced stroke risk and concluded that after adjusting age, smoke status, clinical cardiovascular events, and high SBP, DBP <70 mmHg was not related to stroke risk. What the authors trying to explain was that DBP is unlikely to predict outcomes independent of individual characteristics. Stensrud et al. detected that DBP <60 mmHg was related to poor outcome risk including stroke (HR, 1.9; 95% CI, 1.46–2.47) and after adjustment, HR improved (HR, 1.04; 95% CI, 0.98–1.10). In further analysis in subjects aged >75 years, no adverse connection was observed (22). Same conclusion has been conducted by two other studies that question the clinical significance of DBP independent of SBP (23,24). Meanwhile, Williams (25) also proposed controversies about DBP being applied to define hypertension and initiate treatment. These studies seem to support the inability of DBP to be used for prediction while emphasizing the importance of other clinical variables. In our study, we analyzed the non-linear relationship between DBP and stroke and DBP <80 mmHg was significantly associated with increased risk of stroke, but the model in multivariate logistic regression did not find a statistically significant relationship between them. One explanation is that adjusting the clinical variables weakens the direct impact of DBP on stroke. Besides, the differences in the study population and individual characteristics of the subjects may also be the cause of these diversities. Perhaps, DBP maybe not as important as SBP in diagnosis and management of hypertension or predicting prognosis currently, but DBP must be essential and it needs more large, randomized and controlled trials to prove itself. Considering that the results of relationship between DBP and stroke differ in different study groups, future studies should be committed to providing different guidance and advice to different groups of people.
There are some limitations needs to be noted in our study. First and most importantly, we only can reveal that DBP was non-linearly relevant to stroke, but it was difficult to distinguish causal relationship between them considering that this study was a retrospective cross-sectional study. Well-designed prospective cohort study is needed to clarify it. Second, regarding stroke is a multivariate related disease, although we consider many confounding factors which are related to the occurrence of stroke in our analysis, we were unable to adjust physical activity due to raw data limitations since the lack of exercise is closely associated with higher stroke risk (26). Third, because this study is a retrospective investigation, the history of stroke mainly comes from patient self-reporting, there may be deviations in reporting; in addition, there may be ambiguous memory, and some patients may have recurrent stroke. Fourth, the population of this study came from a single center of the Chinese population, so the conclusions of the study cannot be extrapolated to other populations and ethnic groups.
Conclusions
In conclusion, our study found a non-linear relationship between DBP and prior stroke in patients with essential hypertension. DBP was negatively correlated with stroke when less than 80 mmHg, suggesting there might be no benefit for lower DBP control. These data might provide evidence on stroke prevention and blood pressure control in the future.
Acknowledgments
We thank all the participants.
Funding: This work was supported by the Science and Technology Plan Project of Guangdong Province (No. 2017B030314041), the Natural Science Foundation of Guangdong Province (No. 2015A030313660), the Science and Technology Program of Guangzhou (No. 201604020143, No. 201604020018, No. 201604020186, No. 201510010254, and No. 201803040012), and the National Key Research and Development Program of China (No. 2017FYC1307603, No. 2016YFC1301305, 2017YFC0909303), and the Key Area R&D Program of Guangdong Province (No. 2019B020227005).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2020.02.04). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Our experimental study was approved by the institutional medical ethical committee the Guangdong General Hospital, Guangzhou, China (No. GDREC2012143H). All participants have provided informed consent in written form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- GBD 2016 Stroke Collaborators. Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019;18:439-58. [Crossref] [PubMed]
- Wu S, Wu B, Liu M, et al. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol 2019;18:394-405. [Crossref] [PubMed]
- Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation 2014;129:e28-292. [PubMed]
- MacMahon S, Peto R, Cutler J, et al. Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet 1990;335:765-74. [Crossref] [PubMed]
- Wright JT Jr, Williamson JD, Whelton PK, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med 2015;373:2103-16. [Crossref] [PubMed]
- Lacey B, Lewington S, Clarke R, et al. Age-specific association between blood pressure and vascular and non-vascular chronic diseases in 0.5 million adults in China: a prospective cohort study. Lancet Glob Health 2018;6:e641-9. [Crossref] [PubMed]
- Voko Z, Bots ML, Hofman A, et al. J-shaped relation between blood pressure and stroke in treated hypertensives. Hypertension 1999;34:1181-5. [Crossref] [PubMed]
- Cruickshank JM, Thorp JM, Zacharias FJ. Benefits and potential harm of lowering high blood pressure. Lancet 1987;1:581-4. [Crossref] [PubMed]
- Banach M, Aronow WS. Blood pressure j-curve: current concepts. Curr Hypertens Rep 2012;14:556-66. [Crossref] [PubMed]
- Vidal-Petiot E, Greenlaw N, Ford I, et al. Relationships Between Components of Blood Pressure and Cardiovascular Events in Patients with Stable Coronary Artery Disease and Hypertension. Hypertension 2018;71:168-76. [Crossref] [PubMed]
- Malyszko J, Muntner P, Rysz J, et al. Blood pressure levels and stroke: J-curve phenomenon? Curr Hypertens Rep 2013;15:575-81. [Crossref] [PubMed]
- Lackland DT, Carey RM, Conforto AB, et al. Implications of Recent Clinical Trials and Hypertension Guidelines on Stroke and Future Cerebrovascular Research. Stroke 2018;49:772-9. [Crossref] [PubMed]
- Liu LS. 2010 Chinese guidelines for the management of hypertension. Zhonghua Xin Xue Guan Bing Za Zhi 2011;39:579-615. [PubMed]
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2018;138:e426-83. [PubMed]
- Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018;39:3021-104. [Crossref] [PubMed]
- Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet 1998;351:1755-62. [Crossref] [PubMed]
- Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;362:1575-85. [Crossref] [PubMed]
- Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA 1991;265:3255-64. [Crossref] [PubMed]
- Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008;358:1887-98. [Crossref] [PubMed]
- Choi YJ, Kim SH, Kang SH, et al. Reconsidering the cut-off diastolic blood pressure for predicting cardiovascular events: a nationwide population-based study from Korea. Eur Heart J 2019;40:724-31. [Crossref] [PubMed]
- Sobieraj P, Lewandowski J, Sinski M, et al. Low Diastolic Blood Pressure is Not Related to Risk of First Episode of Stroke in a High-Risk Population: A Secondary Analysis of SPRINT. J Am Heart Assoc 2019;8:e010811. [Crossref] [PubMed]
- Stensrud MJ, Strohmaier S. Diastolic hypotension due to intensive blood pressure therapy: Is it harmful? Atherosclerosis 2017;265:29-34. [Crossref] [PubMed]
- Benetos A, Thomas F, Bean K, et al. Prognostic value of systolic and diastolic blood pressure in treated hypertensive men. Arch Intern Med 2002;162:577-81. [Crossref] [PubMed]
- Lindenstrom E, Boysen G, Nyboe J. Influence of systolic and diastolic blood pressure on stroke risk: a prospective observational study. Am J Epidemiol 1995;142:1279-90. [Crossref] [PubMed]
- Williams B, Lindholm LH, Sever P. Systolic pressure is all that matters. Lancet 2008;371:2219-21. [Crossref] [PubMed]
- O'Donnell MJ, Xavier D, Liu L, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet 2010;376:112-23. [Crossref] [PubMed]
Cite this article as: Chen CL, Liu L, Huang YQ, Shen G, Huang JY, Yu YL, Tang ST, Chen JY. Association of diastolic blood pressure with history of stroke in community hypertensive patients: a cross-sectional study. J Xiangya Med 2020;5:5.



