
Prevalence and associated factors of hypokalemia in hypertension: the perspective in a low to middle-income setting
Introduction
Hypertension is a major cardiovascular risk factor accounting for about 50% of cardiovascular deaths globally (1,2). Compared to high-income countries, the rate of hypertension has been on the rise in low to middle-income settings (3). Effective treatment of this condition warrants adequate lifestyle modifications as well as pharmacologic management (4,5). Pharmacologic management of hypertension has well-established adverse effects of which electrolyte disturbance are amongst. Hypokalemia defined as serum potassium less than 3.5 mmol/L (6-8) is a well-established adverse effect of pharmacologic management of diuretic therapy (9,10) which remains the cornerstone in the management of hypertension (4,11). Moreover, hypokalemia in hypertension can as well be present in specific etiologies of hypertension with the most common being primary aldosteronism (12-14), with recent endocrine society guidelines recommending screening for primary aldosteronism in any case of hypertension presenting with spontaneous or diuretic-induced hypokalemia (12,13).
Several studies have demonstrated the relationship between potassium and blood pressure (BP) (7,15-19) and although serum potassium is not an absolute predictor of total body potassium, studies have determined a U-shaped relationship between cardiovascular outcomes and serum potassium (8,20-22). Compared to Caucasian populations, studies have reported salt sensitivity in the black populations, these studies further demonstrated blunting of the sodium effect on BP once potassium intake is increased (23). Furthermore, data reports low urinary potassium excretion in blacks when compared to Caucasians regardless of potassium intake (24,25).
To our knowledge, most studies reporting the prevalence of hypokalemia in hypertension were carried out in high-income countries, with these studies revealing the prevalence of hypokalemia in hypertension between 6–21% (26,27). Thus, we carried a study to measure the prevalence of hypokalemia in hypertension as well as the factors associated with hypokalemia in hypertension in a low to middle-income setting.
Methods
Study design and setting
This was a 5-year retrospective (January 2014–January 2019) observational study conducted at the outpatient department of Douala General Hospital (DGH) which is a tertiary hospital located in the urban city of Douala. Data were obtained from the DGH hypertension registry, an ongoing registry created by cardiologists of the DGH since 2010.
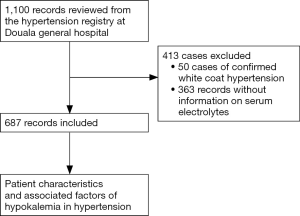
Records with confirmed hypertension by an attending cardiologist (based on office BP ≥140/90 mmHg or 24-hour ambulatory BP ≥130/80 mmHg) (28) were included, while records with confirmed white coat hypertension (records with office BP ≥140/90 mmHg with 24-hour ambulatory BP <130/80 mmHg) (28), and records with no information on serum electrolyte were excluded from the study.
Data collection
Administrative authorization was obtained from the central administration of the DGH and ethical clearance from the Faculty of Health Sciences University of Bamenda, Cameroon.
Blood pressure: BP was obtained from patient records which were measured following the European Society of Cardiology (ESC) 2013 guidelines (28) and the average BP for each patient record was obtained by summing the second and third BP reading in each record and dividing by two.
Serum potassium and other laboratory investigations were obtained from patient records, information concerning usage of antihypertensive medications was obtained during the first consultation and those reported to be newly diagnosed with hypertension were considered not to be on any medications. Hypokalemia was considered as serum potassium <3.5 mmol/L. Mild, moderate and severe hypokalemia was defined as serum potassium between 3–3.4, 2.5–2.9 and <2.5 mmol/L respectively. Figure 1 summarizes the steps for the recruitment of study participants.
Statistical analyses
Data obtained from patient records were analyzed using SPSS version 25 for windows, patients were classified into three groups based on serum potassium. Categorical variables were presented as frequencies and percentages while continuous variables were presented as mean and standard deviation. Categorical variables were compared using the Chi-squared (χ2) test and Fischer exact test whereas one-way analysis of variance was used to compare continuous variables, Brown-Forsythe correction was used to adjust P values for continuous variables which violated the Levene’s test for homogeneity of variance. Univariate and multivariate logistic regression was used to determine the associated factors of hypokalemia in hypertension with the calculation of odds ratio (OR) and 95% confidence intervals (CIs). A P value of less than 0.05 was considered significant.
Results
Patient characteristics
In total, 687 participants were included in the study. The demographic and clinical characteristics of these participants are summarized in Table 1 based on three levels of serum potassium. Overall 62% [427] of the participants were females with a mean age of 57.5±12.9 years. The mean systolic BP (SBP) and diastolic BP (DBP) were 163.8±24.0 and 95.8±16.0 mmHg respectively with 65% of participants reported having either grade 1 or grade 2 hypertension based on the ESC classification. Also, 74% of participants reported being on BP-lowering medications. The mean serum potassium was 3.9±0.55 mmol/L with 71% of participants reported to have serum potassium between 3.5–5.0 mmol/L.
Table 1
| Variables | K <3.5 mmol/L, n=180 | K 3.5–5 mmol/L, n=488 | K >5 mmol/L, n=19 | P value |
|---|---|---|---|---|
| Female | 114 (63.3) | 302 (61.9) | 11 (57.9) | 0.88 |
| Age (years), (SD) | 57.67 (12.63) | 57.45 (13.02) | 58.42 (13.50) | 0.94 |
| Risk factors | ||||
| Diabetes mellitus | 19 (10.6) | 53 (10.9) | 2 (10.5) | 0.99 |
| Heart failure | 7 (3.9) | 13 (2.7) | 0 | 0.53 |
| Tobacco | 4 (2.2) | 14 (2.9) | 1 (5.3) | 0.72 |
| Signs and symptoms | ||||
| Palpitations | 23 (12.8) | 78 (15.9) | 1 (5.3) | 0.29 |
| Clinical parameters | ||||
| Weight (kg) | 80.06 (14.20) | 80.54 (15.02) | 77.42 (15.12) | 0.64 |
| Heart rate (bpm) | 79.39 (14.36) | 79.58 (14.49) | 77.21 (17.43) | 0.78 |
| SBP (mmHg) | 168.08 (25.63) | 163.94 (22.99) | 185.05 (24.83) | <0.001 |
| DBP (mmHg) | 95.19 (16.42) | 95.84 (15.78) | 100.13 (16.21) | 0.44 |
| Laboratory parameters | ||||
| Sodium (mmol/L) | 137.58 (6.55) | 139.13 (6.91) | 142.11 (11.34) | 0.004 |
| Creatinine (mg/dL) | 1.02 (0.37) | 1.15 (1.0) | 2.16 (2.48) | 0.05 |
| eGFR (mL/min/1.73 m2) | 87.56 (26.63) | 88.90 (29.60) | 72.61 (36.16) | 0.06 |
| LDL (g/L) | 1.28 (0.43) | 1.36 (0.46) | 1.23 (0.62) | 0.32 |
| Total cholesterol (g/L) | 1.96 (0.49) | 2.10 (0.50) | 1.95 (0.79) | 0.02 |
| Serum glucose (mg/dL) | 99.61 (25.38) | 99.36 (37.49) | 105.43 (25.75) | 0.81 |
| Medications | ||||
| On blood pressure lowering medications | 155 (86.1) | 342 (70.1) | 14 (73.9) | <0.001 |
| Beta blockers | 14 (7.7) | 46 (9.4) | 1 (5.3) | 0.32 |
| CCBs | 91 (50.6) | 193 (39.5) | 7 (36.8) | 0.77 |
| ACE inhibitors | 77 (42.8) | 189 (38.7) | 8 (42.1) | 0.51 |
| ARBs | 20 (11.1) | 38 (7.8) | 0 | 0.33 |
| Diuretics | 128 (71.1) | 227 (46.5) | 6 (31.6) | <0.001 |
| ACEi + diuretics | 71 (39.4) | 96 (19.8) | 2 (10.5) | <0.001 |
ACEi, angiotensin converting enzyme inhibitor; ARBs, angiotensin receptor blockers; bpm, beats per minute; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; K, serum potassium; LDL, low density lipoproteins; SBP, systolic blood pressure; CCBs, calcium channel blockers.
Prevalence of hypokalemia in hypertension
Of the 687 records reviewed, 180 had serum potassium less than 3.5 mmol/L thus the prevalence of 26.2% (95% CI, 22.9–29.7%). Out of these 180 cases, 82.8% were cases of mild hypokalemia, 16.7% had moderate hypokalemia and 0.6% were cases of severe hypokalemia.
Factors associated with hypokalemia
On univariate analysis, use of calcium channel blockers (CCBs) (OR =1.57, P<0.05) was associated with hypokalemia, also use of diuretics was associated with hypokalemia with use of indapamide (OR =5.7, P<0.001) associated more with hypokalemia than hydrochlorothiazide (OR =1.6, P=0.01). Female sex had a decreased likelihood to present with hypokalemia (OR =0.93, P=0.70) but this was however not statistically significant. All variables used for univariate analysis were entered into the multivariate model, the use of diuretics was significantly associated with hypokalemia, with participants using thiazide-like diuretics (indapamide and chlorthalidone) presenting higher chances of hypokalemia than those using of thiazide-type diuretics (hydrochlorothiazide). Table 2 summarizes the results obtained from univariate and multivariate logistic regression.
Table 2
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | aOR (95% CI) | P value | ||
| Female sex | 0.93 (0.66–1.33) | 0.70 | 1.11 (0.77–1.62) | 0.57 | |
| Age (years) | 1.00 (0.98–1.01) | 0.87 | 0.99 (0.98–1.01) | 0.92 | |
| Hydrochlorothiazide | 1.6 (1.22–2.84) | 0.004 | 1.92 (1.15–3.22) | 0.01 | |
| Chlorthalidone | 4.38 (1.29–14.87) | 0.02 | 4.82 (1.32–17.52) | 0.02 | |
| Indapamide | 5.70 (3.59–9.07) | <0.001 | 5.89 (3.52–9.87) | <0.001 | |
| Furosemide | 1.75 (0.46–6.68) | 0.41 | 2.17 (0.54–8.78) | 0.27 | |
| CCBs (yes) | 1.57 (1.12–2.21) | 0.01 | 1.16 (0.79–1.70) | 0.44 | |
| Beta blockers (yes) | 0.83 (0.44–1.54) | 0.55 | 0.79 (0.39–1.57) | 0.50 | |
| ARBs (yes) | 1.54 (0.87–2.73) | 0.14 | 1.66 (0.85–3.27) | 0.14 | |
| ACE inhibitors (yes) | 1.18 (0.83–1.66) | 0.34 | 0.85 (0.53–1.34) | 0.48 | |
| Fasting plasma glycemia >100 (mg/dL) | 1.36 (0.95–1.95) | 0.09 | 1.26 (0.86–1.85) | 0.24 | |
| eGFR <60 (mL/min/1.73 m2) | 0.93 (0.57–1.53) | 0.77 | 0.84 (0.49–1.44) | 0.52 | |
ACE, angiotensin converting enzyme; ARBs, angiotensin receptor blockers; aOR, adjusted odds ratio; CCBs, calcium channel blockers; CI, confidence interval; eGFR, estimated glomerular filtration rate; OR, odds ratio.
Discussion
In this study, we demonstrated a high prevalence of hypokalemia (26.2%) in a black African hypertensive population, with use of diuretics independently associated with hypokalemia. Furthermore, participants receiving thiazide-like (indapamide and chlorthalidone) diuretics were more likely to present with hypokalemia than those on thiazide-type (hydrochlorothiazide) diuretics. To our knowledge, this is the first study reporting the prevalence of hypokalemia in hypertension in the general hypertensive population in a sub-Saharan African setting.
Prevalence of hypokalemia in hypertension
Most studies reporting the prevalence of hypokalemia in hypertension are focused on the diagnosis of primary aldosteronism with most of the participants presenting with specific characteristics like resistant hypertension or severe hypertension (26,27). This study included all categories of patients thus representing a general hypertensive cohort. The prevalence of hypokalemia in this study was 26.2%, similar to that observed by Douma et al. (26). Although the Douma series included only participants with resistant hypertension nevertheless, Monticone and colleagues reported a lower prevalence (6.3%) (27). Notably, this was a study to report the prevalence of primary aldosteronism and antihypertensive medications in most participants were withheld for 4–6 weeks prior to measurement of serum electrolytes and aldosterone renin ratio.
Factors associated with hypokalemia
Diuretics are strongly recommended by studies and hypertension guidelines as first-line agents in the treatment of hypertension, especially in blacks (4,5,29). In addition, studies have established superiority of thiazide-like diuretics (chlorthalidone and indapamide) over thiazide-type diuretics (hydrochlorothiazide) with regards to BP-lowering and cardiovascular risk protection, with similar effects on metabolic profile (serum electrolytes and serum glucose) (11,30,31). Nonetheless, thiazide diuretics are associated with hypokalemia (9). This study revealed diuretics were independently associated with hypokalemia in hypertension and on further analyses, chlorthalidone and indapamide were more associated with hypokalemia than hydrochlorothiazide. These results are in agreement with findings of a systematic review conducted by Dorsch et al. where chlorthalidone was associated with more hypokalemia than hydrochlorothiazide (11). However, they are somehow different from those observed in other systematic reviews, which included studies conducted almost exclusively in Caucasians and where there was no difference between thiazide-like and thiazide-type diuretics on serum potassium levels (11,30). These contrasting findings can be explained by racial or nutritional differences. It shall be noted that some studies have shown that urinary potassium excretion is less in blacks than Caucasians even when identical amounts of potassium are provided in the diet (24,25). However, because the urinary potassium excretion also depends on sodium intake and diet, we cannot rule out the possibility that a low salt diet which is largely prescribed by physicians in our setting might have influenced plasma potassium levels in our population. Also, potassium depletion has been reported to increase mean arterial BP (32), through pressor effects and alteration of vascular resistance, in addition, it is as well reported that potassium depletion increases urinary calcium excretion which has been stipulated by several studies to have positive pressor effects. Finally, that a large proportion of our population was on angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) could have resulted in less potassium excretion due to a synergistic effect of these medications with diuretics. This has been particularly advocated and demonstrated in populations with low-renin hypertension (33,34). Patients with essential hypertension especially those with resistant hypertension are generally reported to have high plasma renin (35) which can, in turn, result in negative potassium balance by subsequent activation of the renin aldosterone angiotensin pathway. However, studies have reported black patients often present with low renin hypertension (36). It is worth noting that hypertension in blacks is salt-sensitive, this effect is however blunted by increased potassium intake (23,37). Also, non-pharmacologic treatment of hypertension encourages patients to adopt the DASH (dietary approach to stop hypertension) diet which has been reported to effectively decrease BP, this diet is also noted for its high potassium and calcium content (38).
Limitations of the study
The present study has some limitations that must be underscored. First, its retrospective design precluded any standardizing methods of measuring serum potassium and the collection of data in all eligible patients as well as reporting the proportion of patients who were on potassium supplements which could have an impact on the prevalence of hypokalemia. Second, in the absence of randomization, there was no head to head comparison of diuretics in this study. Third, diuretics does not only reduced potassium, but also increased uric acid and increase total cholesterol and triglycerides but we did not assess other metabolic changes.
Despite these short comes, our study is one of the rare studies evaluating the prevalence and associated factors of hypokalemia in a general hypertensive cohort in a low to middle-income setting, our findings provide baseline values for planning future research on hypertension in our setting.
Conclusions
This study revealed 1 out of 4 patients with hypokalemia, and though not randomized patients on thiazide-like diuretics (indapamide and chlorthalidone), were more likely to present with hypokalemia than those on thiazide-type diuretics (hydrochlorothiazide). More attention should be paid on serum electrolytes of patients with hypertension and consideration should be given to promoting the intake of potassium-rich foods, as this may balance the consequences of hypokalemia.
Acknowledgments
The authors wish to thank all doctors and nurses in charge of the hypertension registry at the Douala General Hospital.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2020.03.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The protocol of this study was approved by the institutional review board of the faculty of health sciences university of Bamenda (2019/0045H/Uba/IRB), and the study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Individual informed consent was waived due to the retrospective nature of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Beaney T, Burrell LM, Castillo RR, et al. May Measurement Month 2018: a pragmatic global screening campaign to raise awareness of blood pressure by the International Society of Hypertension. Eur Heart J 2019;40:2006-17. [Crossref] [PubMed]
- World Health Organization. A global brief on hypertension: silent killer, global public health crisis: World Health Day 2013. Geneva: World Health Organization, 2013.
- Ogah OS, Rayner BL. Recent advances in hypertension in sub-Saharan Africa. Heart 2013;99:1390-7. [Crossref] [PubMed]
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018;71:1269-324. [Crossref] [PubMed]
- Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur Heart J 2018;39:3021-104. [Crossref] [PubMed]
- Gumz ML, Rabinowitz L, Wingo CS. An integrated view of potassium homeostasis. N Engl J Med 2015;373:60-72. [Crossref] [PubMed]
- McDonough AA, Youn JH. Potassium homeostasis: the knowns, the unknowns, and the health benefits. Physiology (Bethesda) 2017;32:100-11. [Crossref] [PubMed]
- Krogager ML, Torp-Pedersen C, Mortensen RN, et al. Short-term mortality risk of serum potassium levels in hypertension: a retrospective analysis of nationwide registry data. Eur Heart J 2017;38:104-12. [PubMed]
- Cooney D, Milfred-LaForest S, Rahman M. Diuretics for hypertension: Hydrochlorothiazide or chlorthalidone? Cleve Clin J Med 2015;82:527-33. [Crossref] [PubMed]
- Rodenburg EM, Visser LE, Hoorn EJ, et al. Thiazides and the risk of hypokalemia in the general population. J Hypertens 2014;32:2092-7; discussion 2097. [Crossref] [PubMed]
- Dorsch MP, Gillespie BW, Erickson SR, et al. Chlorthalidone reduces cardiovascular events compared with hydrochlorothiazide: a retrospective cohort analysis. Hypertension 2011;57:689-94. [Crossref] [PubMed]
- Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2016;101:1889-916. [Crossref] [PubMed]
- Young WF. Diagnosis and management of primary aldosteronism. Cham: Adrenal Disorders. Humana Press, 2018:245-60.
- Satoh M, Maruhashi T, Yoshida Y, et al. Systematic review of the clinical outcomes of mineralocorticoid receptor antagonist treatment versus adrenalectomy in patients with primary aldosteronism. Hypertens Res 2019;42:817-24. [Crossref] [PubMed]
- Perez V, Chang ET. Sodium-to-potassium ratio and blood pressure, hypertension, and related factors. Adv Nutr 2014;5:712-41. [Crossref] [PubMed]
- Stone MS, Martyn L, Weaver CM. Potassium intake, bioavailability, hypertension, and glucose control. Nutrients 2016;8:444. [Crossref] [PubMed]
- Poorolajal J, Zeraati F, Soltanian AR, et al. Oral potassium supplementation for management of essential hypertension: a meta-analysis of randomized controlled trials. PLoS One 2017;12:e0174967. [Crossref] [PubMed]
- Sebastian A, Cordain L, Frassetto L, et al. Postulating the major environmental condition resulting in the expression of essential hypertension and its associated cardiovascular diseases: Dietary imprudence in daily selection of foods in respect of their potassium and sodium content resulting in oxidative stress-induced dysfunction of the vascular endothelium, vascular smooth muscle, and perivascular tissues. Med Hypotheses 2018;119:110-9. [Crossref] [PubMed]
- Ekmekcioglu C, Elmadfa I, Meyer AL, et al. The role of dietary potassium in hypertension and diabetes. J Physiol Biochem 2016;72:93-106. [Crossref] [PubMed]
- Aburto NJ, Hanson S, Gutierrez H, et al. Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta-analyses. BMJ 2013;346:f1378. [Crossref] [PubMed]
- Luo J, Brunelli SM, Jensen DE, et al. Association between serum potassium and outcomes in patients with reduced kidney function. Clin J Am Soc Nephrol 2016;11:90-100. [Crossref] [PubMed]
- Hughes-Austin JM, Rifkin DE, Beben T, et al. The relation of serum potassium concentration with cardiovascular events and mortality in community-living individuals. Clin J Am Soc Nephrol 2017;12:245-52. [Crossref] [PubMed]
- Cappuccio FP, MacGregor GA. Does potassium supplementation lower blood pressure? A meta-analysis of published trials. J Hypertens 1991;9:465-73. [Crossref] [PubMed]
- Voors AW, Dalferes ER Jr, Frank GC, et al. Relation between ingested potassium and sodium balance in young Blacks and whites. Am J Clin Nutr 1983;37:583-94. [Crossref] [PubMed]
- Barlow RJ, Connell MA, Milne FJ. A study of 48-hour faecal and urinary electrolyte excretion in normotensive black and white South African males. J Hypertens 1986;4:197-200. [Crossref] [PubMed]
- Douma S, Petidis K, Doumas M, et al. Prevalence of primary hyperaldosteronism in resistant hypertension: a retrospective observational study. Lancet 2008;371:1921-6. [Crossref] [PubMed]
- Monticone S, Burrello J, Tizzani D, et al. Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J Am Coll Cardiol 2017;69:1811-20. [Crossref] [PubMed]
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013;34:2159-219. [Crossref] [PubMed]
- Dzudie A, Rayner B, Ojji D, et al. Roadmap to achieve 25% hypertension control in Africa by 2025. Cardiovasc J Afr 2017;28:262-72. [Crossref] [PubMed]
- Liang W, Ma H, Cao L, et al. Comparison of thiazide-like diuretics versus thiazide-type diuretics: a meta-analysis. J Cell Mol Med 2017;21:2634-42. [Crossref] [PubMed]
- Barrios V, Escobar C. Which thiazide to choose as add-on therapy for hypertension? Integr Blood Press Control 2014;7:35-47. [Crossref] [PubMed]
- Coruzzi P, Brambilla L, Brambilla V, et al. Potassium depletion and salt sensitivity in essential hypertension. J Clin Endocrinol Metab 2001;86:2857-62. [Crossref] [PubMed]
- Holland OB, von Kuhnert L, Campbell WB, et al. Synergistic effect of captopril with hydrochlorothiazide for the treatment of low-renin hypertensive black patients. Hypertension 1983;5:235-9. [Crossref] [PubMed]
- Destro M, Cagnoni F, D'Ospina A, et al. New strategies and drugs in the treatment of hypertension: monotherapy or combination? Recent Pat Cardiovasc Drug Discov 2010;5:69-81. [Crossref] [PubMed]
- Gordon DB. The role of renin substrate in hypertension. Hypertension 1983;5:353-62. [Crossref] [PubMed]
- Spence JD, Rayner BL. Hypertension in blacks: individualized therapy based on renin/aldosterone phenotyping. Hypertension 2018;72:263-9. [Crossref] [PubMed]
- Falkner B. Does potassium deficiency contribute to hypertension in children and adolescents? Curr Hypertens Rep 2017;19:37. [Crossref] [PubMed]
- Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 1997;336:1117-24. [Crossref] [PubMed]
Cite this article as: Barche B, Dzudie A, Moor VA, Azabji MK, Stanis F, Messaline F, Peter E, Mouliom S, Felicite K, Halle MP, Etoundi Ngoa LS, Ashuntantang G. Prevalence and associated factors of hypokalemia in hypertension: the perspective in a low to middle-income setting. J Xiangya Med 2020;5:34.


