
High-resolution chest CT angiography of patients with COVID-19 pneumonia: a longitudinal prospective study
Introduction
More than 68 million people received the diagnosis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and more than 1.5 million persons died with the diagnosis of coronavirus disease 2019 (COVID-19) all over the world at the time of writing. Chest CT shows typical, though non-specific, features and has been used for diagnostic purposes (1-5) as well as to follow-up (6,7) and to predict clinical outcome (8,9).
Postmortem biopsies of patients with SARS-CoV-2 have shown diffuse alveolar damage, cellular exudates, hyaline membrane formation, thickening of intra- and inter-lobular septa and interstitial mononuclear inflammatory infiltrates mainly represented by lymphocytes (10,11). These features are currently considered the pathological correlates of ground-glass opacifications, crazy paving pattern and consolidations detected on chest CT images (1-5). Microscopic analysis of autopsy samples further demonstrated CD4+ lymphocytes aggregated around small vessels, some containing platelets and fibrin thrombi, with vascular wall thickening, vascular congestion and foci of hemorrhage (11,12). Moreover, histologic analysis of pulmonary vessels revealed diffuse thrombosis and microangiopathy in patients with COVID-19 (13).
Critical intensive care unit (ICU) patients with COVID-19 have also high frequency of thrombotic complications (14). Chest CT angiography (CTA) recently showed high prevalence of acute pulmonary embolism in patients with COVID-19 at a mean of 12 days from symptom onset (15).
Free-breathing 384-row detector CTA with high spatial resolution is not widely available in emergency settings. However, the assessment of critical patients and the evaluation of segmental and subsegmental pulmonary vessels may benefit from the state-of-the-art technology, which possibly may capture the full vascular picture of COVID-19. Moreover, the relationship of CT and CTA features with arterial blood gas (ABG) parameters, as indicators of alveolar exchange, is so far not fully understood.
The aims of this study were to quantitatively analyze pulmonary disease burden on chest CT, to assess pulmonary vessel involvement on 384-row state-of-the-art chest CTA images in patients with COVID-19 pneumonia and integrate these data with ABG analysis.
We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/jxym-20-109).
Methods
Study design
We prospectively assessed patients admitted to the Covid Center of our hospital between April 1st and April 15th 2020. In particular, we longitudinally collected clinical, laboratory and radiological data at the time of hospital admission (day 0) and within the 7 days after hospitalization.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics committee of Università Campus Bio-Medico di Roma (Prot.: 88/20 OSS.NOT ComEt CBM) and informed consent was taken from all the patients.
Patients
Patients were admitted to ICU according to the following criteria: COVID-19 with positive reverse transcriptase polymerase chain reaction (RT-PCR) for SARS-CoV-2 and need for invasive and non-invasive ventilation based on the clinical status. Patients with COVID-19 not requiring ventilation were admitted to the low-cure Covid Medicine area of the hospital (non-ICU). Patients admitted to non-ICU area were symptomatic on room air and required supplemental oxygen.
Since the day of admission, a thromboprophylaxis regimen with enoxaparin was established as it follows: for non-ICU patients with prophylactic daily dose of ≤40 mg a day and for ICU patients with therapeutic dose of >40 mg a day. The therapy was subsequently adjusted on a case-by-case clinical basis.
The ABG analysis was used for monitoring the patients. The following parameters were recorded: pO2, pCO2, FIO2, P/F ratio, SO2, lactates, pH and FO2 Hb. P/F ratio equals the arterial pO2 divided by the FIO2. P/F ratio is commonly used to identify acute hypoxemic respiratory failure when supplemental oxygen is administered. Values of P/F ratio >400 are typically normal. The severity of respiratory distress was considered to be mild with P/F ratio of 200–300, moderate with P/F ratio of 100–200 and severe with P/F ratio <100.
All patients underwent a non-contrast chest CT scan at the admission (day 0) and repeated the chest CT scan with or without CT angiography within the first 7 days of hospitalization on a case-by-case basis, according to the clinical needs.
CT protocol and image analysis
Chest CT images were acquired with Dual Source 384-slice (2×192) CT scanner {Siemens SOMATOM Force [tube voltage: 100 kV; tube real-time dose modulation (CARE Dose4DTM) 80–250 mAs; spiral pitch factor: 1.8; collimation width: 0.6 mm]}.
CTA was performed after intravenous injection of 80 mL iodinated contrast agent (Omnipaque 350 mgI/mL, GE Healthcare) at a flow rate of 5 mL/sec through the automatic injector (Medrad Stellant CWS, Bayer Healthcare), and using the main pulmonary artery as trigger (triggering threshold at 80 HU) for image acquisition [tube voltage: 150 kV; tube real-time dose modulation (CARE Dose4DTM) 80–250 mAs; spiral pitch factor: 2.5; collimation width: 0.6 mm].
DICOM data were transferred to a dedicated platform for automatic detection and segmentation of lung lesions InferReadTM CT Lung (COVID-19) (Infervision, Europe GmbH, Wiesbaden, Germany), an artificial intelligence (AI) solution specifically developed for support to the diagnosis and management of COVID-19 pneumonia (16). The quantitative assessment of chest CT images yielded the following parameters: the volume of lesions (total and lobar), the relative volume percentage of lesions in the lungs and each lobe volume and the severity score based on the percentages as explained elsewhere (8).
Three radiologists (PDA, CCQ, and CAM, with 19, 16 and 9 years of experience respectively) assessed the vascular involvement by simultaneous reading, discussion and consensus on a PACS workstation (Philips Healthcare Information Solutions). Coronal and maximum intensity projections (MIPs, thickness 10–20 mm) formatted images were assessed for the detection of: (I) pulmonary embolism (i.e., intraluminal filling defect), (II) stenosis and vascular wall thickening/irregularity of segmental and/or subsegmental branches of pulmonary artery; (III) tubular dilation (>3 mm) of subsegmental branches of pulmonary artery and (IV) focal dilation of subsegmental branches of pulmonary artery.
Statistical analysis
For statistical purposes, patients were divided into two groups (ICU and non-ICU) at day 0 based on the level of care and in two groups (CTA and non-CTA) at follow-up after admission, based on the need for contrast chest CTA. Descriptive statistics, including means and standard deviations were calculated to understand central tendencies of the samples. Data distribution normality was checked by means of Kolmogorov-Smirnov test. The Levene’s test was used to assess the equality of variances. The Fisher exact test was used to compare age and sex distribution among groups. Statistical significance (threshold at P<0.05) of comparisons between groups (Mann-Whitney U test) and of non-parametric correlation between variables (Spearman’s Rho) was obtained by using the Statistical Package for the Social Sciences (SPSS) software version 26.0 (IBM).
Results
Out of the 36 patients referred to the Covid Center, 30.6% (11/36) were admitted to the ICU and 69.4% (25/36) were hospitalized in the non-ICU low-cure Covid Medicine (Figure 1). In this population, symptoms at admission were fever (17/36, 47.2%), cough (12/36, 33.3%), dyspnea (8/36, 22.2%), myalgia and/or arthralgia (5/36, 13.9%), sore throat (3/36, 8.3%) and non-specific gastrointestinal symptoms (3/36, 8.3%). The flow chart of the study and the frequency of comorbidities is shown in Figure 1.
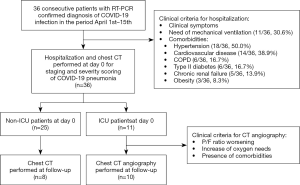
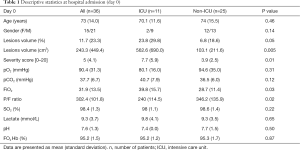
Table 1 shows the age and gender of the patient population, chest CT severity score and lung volume assessment with automatic detection and quantitative output of AI software and ABG at the time of admission (day 0).
Full table
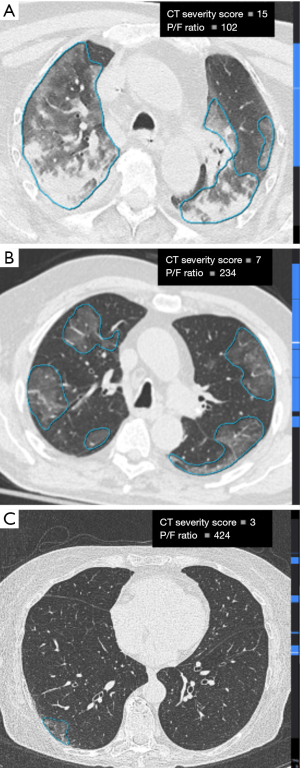
All chest CT scans showed the known features of COVID-19 pneumonia including peripheral, bilateral and multilobar ground glass opacities, with or without crazy paving pattern and in some cases with consolidations. These features were automatically detected and segmented by the AI software (Figure 2).
The chest CT severity score, lesion volume relative percentage and absolute volume (cm3) were significantly higher in the ICU group than in the non-ICU group (P=0.01, P=0.05, P=0.005 respectively). Among ABG parameters, ICU group showed significantly higher FiO2 and lower P/F ratio than non-ICU group (P=0.03 and P=0.02, respectively). No other statistically significant differences were found between the groups (Table 1).
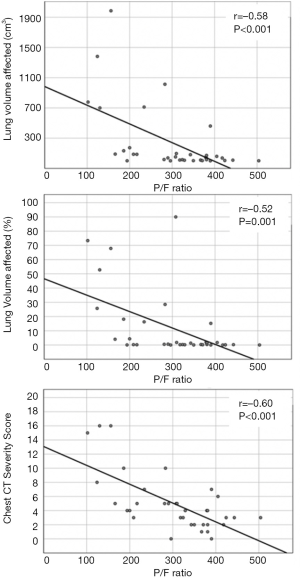
At day 0, there was a significant inverse relationship between the P/F ratio and absolute lesion volume (r=–0.58; 95% CI: –0.76, –0.31; P<0.001), lung lesion volume relative percentages (r=–0.52; 95% CI: –0.72, –0.23; P=0.001) and severity score (r=–0.60; 95% CI: –0.77, –0.34; P<0.001). Examples of the inverse relationship between lung lesion burden on axial chest CT images and the corresponding measured P/F ratio are presented in Figure 3.
At follow-up within 7 days, male and older patients underwent CTA more frequently than females and younger patients (P<0.01 and P=0.02 respectively). Chest CT severity score, lesion volume relative percentage and absolute volume (cm3) did not show significant difference between the CTA group and non-CTA group (P=0.42, P=0.34, P=0.05 respectively). Among ABG parameters, P/F ratio only was significantly lower in the CTA group (P<0.001) (Table 2). Of the 18 patients assessed at follow-up, 39% (7/18) showed mismatch between chest CT pattern and P/F ratio, driven by reduction of lesion volume and severity score despite worsening of the P/F ratio.

Full table
All the patients (10/10) evaluated with CTA were patients admitted to the ICU.
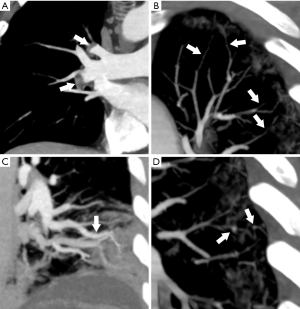
CTA showed pulmonary embolism in 2 out of 10 patients (20%). Moreover, in 9 out of 10 patients (90%) segmental and/or subsegmental branches of pulmonary artery stenosis and vascular wall thickening/irregularity with focal luminal occlusions were present. CTA demonstrated subsegmental tubular vessel dilation in all patients (100%) and subsegmental focal vessel dilations in 6 out of 10 patients (60%) (Figure 4).
Discussion
This study elucidates the full spectrum of vascular pathology in patients with COVID-19 by means of 384-row chest CTA. Moreover, the present study helps to understand the relationship between the quantitative pulmonary disease burden and the vascular involvement of COVID-19 patients with worsening P/F ratio.
Here, a spectrum of four different vascular findings associated to COVID-19 pneumonia is shown. Interestingly, almost all the patients investigated with chest CTA showed segmental and/or subsegmental branches of pulmonary artery stenosis and vascular wall thickening/irregularity with focal luminal occlusions. These abnormalities, that were not systematically investigated with 384-row CTA to the best of our knowledge, are likely to be the counterpart of vascular wall thickening, fibrin thrombi and vascular congestion due to local inflammatory changes, reported by pathology studies (11,12).
In our experience, intraluminal filling defects due to pulmonary embolism were present in 20% (2/10) of the patients. On this respect, we want to emphasize that this is a different entity compared to the aforementioned, since it relates to thrombus migration through the bloodstream with consequent pulmonary thromboembolism. Indeed, thromboembolic risk, coagulopathy and anti-phospholipids antibodies have been described in patients with COVID-19 (17). The presence of acute pulmonary embolism has been reported with contrast-enhanced CT in about 23% of COVID-19 patients with severe clinical picture (15) and associated to high D-Dimer values (18). However, a direct comparison with other studied should be cautious since pulmonary thromboembolism and pulmonary intra-vascular local thrombosis, possibly coexisting in COVID-19, might be easily confused especially when images quality is suboptimal. Indeed, the terms MicroCLOTS (microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome) or COVID-19 associated pulmonary thrombosis, have been proposed to describe an inflammatory reaction and microvascular pulmonary thrombosis (Figures 4,5) that might be associated to COVID-19 (19,20).
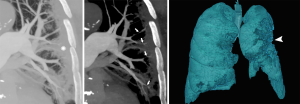
Additionally, we found two different patterns of vascular dilation. Subsegmental tubular vessel dilations were present in all cases. This finding, of unclear etiology and possibly reflecting vasodilation due to local inflammatory processes, has been previously reported in a study by Caruso et al. (2). Of note, we also found focal subsegmental vessel dilations, mostly located at the level of COVID-19 consolidations (Figures 4,5). A similar finding, referred as “microvascular dilation sign”, has been reported by Zhou et al. (21) in 45.2% of the patients with COVID-19. These focal dilations might be the consequence of vessel wall weakening and disruption. In support of this hypothesis, it has been shown that SARS-CoV-2 can produce a direct infection of the endothelial cells and endothelial inflammation (i.e., endotheliitis), likely via a mechanism mediated by angiotensin converting enzyme 2 (ACE2) receptors (22).
Interestingly, the P/F ratio at the time of admission showed a strong association with the quantitative assessment of lung lesions, as evaluated by AI using a deep learning algorithm (23). At follow-up stable low or reduced P/F ratios, a sign of therapy failure, was frequently associated with vascular abnormalities at CTA, including segmental and/or subsegmental vessel dilations and stenoses. Thus, chest CTA is a helpful tool to monitor the patients with severe COVID-19 pneumonia.
Furthermore, we observed a mismatch between CT quantitative volume assessment and P/F ratio in 39% of the patients, meaning that a stable low or worse P/F ratio at follow-up did not match with an improvement of the aerated lung volume.
Although this is a small sample study, our results offer the opportunity to consider that stenoses of segmental and/or subsegmental branches of pulmonary artery due to viral-mediated micro-angiopathic process can explain the lack of treatment efficacy in some patients.
Conclusions
In conclusion, 384-row chest CTA is able to capture the full spectrum of vascular pathology in COVID-19, comprising pulmonary embolism and stenoses together with tubular and focal dilations of segmental and/or subsegmental branches of pulmonary artery. Follow-up CTA would be helpful in order to understand the long-term natural history and clinical significance of these vascular abnormalities.
Acknowledgments
We are extremely grateful to all the clinical and radiological staff of the Campus Covid Center and in particular to De Angelis Alessandro, Lattanzi Lorenzo, Giordano Francesco M., Cristofano Flavia, Dell’Unto Chiara, Fugger Solange, Gilardi Emanuele, Grandi Tommaso, Lelli Diana, Navarin Silvia, Orrù Michela, Beomonte Zobel Bruno, Petrosillo Nicola, Sambuco Federica, Travaglino Francesco, Venturi Marco, Zoccoli Giada, Subioli Stefano, Tomaselli Eleonora, Grasselli Claudia, Lavorante Fedra, Longo Ferdinando, Rigoli Alessandra, Di Pumpo Annalaura, Claps Francesca, and to all the members of the COVID-19 Task Force of our hospital.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/jxym-20-109
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jxym-20-109
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym-20-109). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics committee of Università Campus Bio-Medico di Roma (Prot.: 88/20 OSS.NOT ComEt CBM) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bai HX, Hsieh B, Xiong Z, et al. Performance of radiologists in differentiating COVID-19 from non-COVID-19 viral pneumonia at chest CT. Radiology 2020;296:E46-54. [Crossref] [PubMed]
- Caruso D, Zerunian M, Polici M, et al. Chest CT features of COVID-19 in Rome, Italy. Radiology 2020;296:E79-85. [Crossref] [PubMed]
- Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology 2020;296:E32-40. [Crossref] [PubMed]
- Fang Y, Zhang H, Xie J, et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology 2020;296:E115-7. [Crossref] [PubMed]
- Chung M, Bernheim A, Mei X, et al. CT imaging features of 2019 novel coronavirus (2019-nCoV). Radiology 2020;295:202-7. [Crossref] [PubMed]
- Pan Y, Guan H, Zhou S, et al. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol 2020;30:3306-9. [Crossref] [PubMed]
- Pan F, Ye T, Sun P, et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19). Radiology 2020;295:715-21. [Crossref] [PubMed]
- Bernheim A, Mei X, Huang M, et al. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology 2020;295:200463 [Crossref] [PubMed]
- Colombi D, Bodini FC, Petrini M, et al. Well-aerated lung on admitting chest CT to predict adverse outcome in COVID-19 pneumonia. Radiology 2020;296:E86-96. [Crossref] [PubMed]
- Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420-2. [Crossref] [PubMed]
- Tian S, Xiong Y, Liu H, et al. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol 2020;33:1007-14. [Crossref] [PubMed]
- Fox SE, Akmatbekov A, Harbert JL, et al. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med 2020;8:681-6. [Crossref] [PubMed]
- Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 2020;383:120-8. [Crossref] [PubMed]
- Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020;191:145-7. [Crossref] [PubMed]
- Grillet F, Behr J, Calame P, et al. Acute pulmonary embolism associated with COVID-19 pneumonia detected with pulmonary CT angiography. Radiology 2020;296:E186-8. [Crossref] [PubMed]
- Quattrocchi CC, Mallio CA, Presti G, et al. The challenge of COVID-19 low disease prevalence for artificial intelligence models: report of 1,610 patients. Quant Imaging Med Surg 2020;10:1891-3. [Crossref] [PubMed]
- Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med 2020;382:e38 [Crossref] [PubMed]
- Léonard-Lorant I, Delabranche X, Séverac F, et al. Acute pulmonary embolism in patients with COVID-19 at CT angiography and relationship to d-dimer levels. Radiology 2020;296:E189-91. [Crossref] [PubMed]
- Ciceri F, Beretta L, Scandroglio AM, et al. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc 2020;22:95-7. [PubMed]
- van Nieuwkoop C. COVID-19 associated pulmonary thrombosis. Thromb Res 2020;191:151. [Crossref] [PubMed]
- Zhou S, Wang Y, Zhu T, et al. CT features of coronavirus disease 2019 (COVID-19) pneumonia in 62 patients in Wuhan, China. AJR Am J Roentgenol 2020;214:1287-94. [Crossref] [PubMed]
- Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020;395:1417-8. [Crossref] [PubMed]
- Mallio CA, Quattrocchi CC, Beomonte Zobel B, et al. Artificial intelligence, chest radiographs, and radiology trainees: a powerful combination to enhance the future of radiologists? Quant Imaging Med Surg 2020; In Press.
Cite this article as: Quattrocchi CC, Mallio CA, Stortoni L, D’Alessio P, Galdino I, Mattei A, Gallì B, Di Giorgio E, Donatiello MG, Agrò FE. High-resolution chest CT angiography of patients with COVID-19 pneumonia: a longitudinal prospective study. J Xiangya Med 2021;6:3.


