
Correlation between circulating vascular endothelial growth factor levels and coronary artery disease and its influence on the establishment of coronary collateral circulation: a systematic review and meta-analysis
Introduction
Coronary artery disease (CAD) is manifested by ischemia, hypoxia, or necrosis of the myocardium, resulting from the narrowing, spasm, or obstruction of the coronary artery lumen by atherosclerosis, has become the primary cause of mortality in China and worldwide. Atherosclerosis is an insidious and long-term pathological process, the pathogenesis of which has been associated with multiple theories, including the inflammatory theory, fatty infiltration theory, and thrombosis theory (1). Recent studies have shown that the vascular endothelium plays an essential role in almost all basic biological vascular functions during both healthy and pathogenic states and is involved in the pathogenesis of atherosclerosis (2). Common cardiovascular risk factors result in the impairment of endothelial function through various complex mechanisms, resulting in unfavorable physiological and vascular changes, such as alterations in vasomotor tone alterations, thrombotic dysfunction, smooth muscle cell proliferation and migration, and leukocyte adhesion and migration (3). Thus, endothelial dysfunction contributes to the development of coronary atherosclerosis. However, endothelial cells also play important roles in the formation of collateral circulation. After coronary occlusion due to atherosclerosis, the endothelial cells of collateral vessels undergo a series of transformations to facilitate the subsequent formation of collateral circulation formation, which presents as swollen and longitudinal bulges on the inner surfaces of small coronary arteries, in contrast with the typical completely flat appearance (4). In addition to morphological transformations, the upregulation of several molecules has been associated with increased cell proliferation and migration (5). Thus, the detection of molecules involved in the biological processes of endothelial cells may potentially be used to predict the functional state of the endothelium and further predict the risk of CAD.
Vascular endothelial growth factor (VEGF) is a homogeneous, dimeric glycoprotein with a molecular weight of approximately 45 kDa. VEGF mediates the formation of new blood vessels during angiogenesis by activating the VEGF receptor. VEGF receptor 2 is expressed on the surface of vascular endothelial cells and exerts mitogen-activating and anti-apoptotic effects through intracellular signal transduction pathways, such as the phospholipase C-gamma (PLC-γ)/protein kinase C (PKC), phosphoinositide 3-kinase (PI3K), and mitogen-activated protein kinase (MAPK) pathways (6). Under physiological conditions, VEGF is involved in the proliferation of vascular endothelial cells and promotes the formation of new blood vessels, which play essential roles in the maintenance of homeostasis for the internal environment because blood vessels transport nutrients to tissues and organs and transport catabolic products. Under pathological conditions, such as during neoplastic diseases (7), VEGF is involved in tumor angiogenesis and is associated with the distant metastasis of tumor cells (8).
During severe coronary atherosclerotic diseases, such as chronic total occlusive (CTO) lesions, angiogenesis can serve as a compensatory mechanism to relieve myocardial ischemia; thus, VEGF likely plays a protective role in CAD; However, atherosclerotic plaques become unstable and increasingly prone to causing cardiovascular events when angiogenesis infiltrates into the plaque causing intra-plaque hemorrhage. Animal experiments have confirmed that the exogenous administration of VEGF resulted in atherosclerotic plaque progression (9). Typically, several anastomotic branches with diameters of 20–350 µm exist between the coronary arteries, which are potential channels between the normal coronary arteries and are not open. When coronary artery stenosis exceeds 70%, these anastomotic branches open and gradually develop into a functional collateral circulation (10). The establishment of collateral circulation includes endothelial cell proliferation, extracellular matrix remodeling, and leukocyte aggregation, and VEGF plays important roles in these processes (11). Therefore, VEGF likely has the potential ability to predict the formation of coronary collateral circulation (CCC).
In this meta-analysis, we selected case-control studies and investigated the relationship between the circulating VEGF and the risk of CADs by comparing the level of VEGF in serum or plasma between the patients which is diagnosed by coronary angio-graphy with coronary stenosis over 50%. Besides, the level of circulating VEGF was compared in patients with different grade of collateral circulation formation according to the Rentrop grading system. We present the following article in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/jxym-21-3).
Methods
This study is based on established methods and followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement for reporting systematic reviews and meta-analyses in health-care interventions.
Source
We systematically searched PubMed, Web of Science, Medline, and the Cochrane Library between 1990 and 2020, using the search strategies: (((((((ischemic heart disease[Title/Abstract]) OR (coronary artery disease[Title/Abstract])) OR (CAD[Title/Abstract])) OR (IHD[Title/Abstract])) OR (coronary atherosclerosis[Title/Abstract])) OR (coronary lesion[Title/Abstract])) AND ((VEGF) OR (vascular endothelium growth factor))), ("vascular endothelial growth factor a"[MeSH Terms] OR "vascular endothelial growth factor a"[All Fields] OR "vegf"[All Fields] OR (("endothelium, vascular"[MeSH Terms] OR ("endothelium"[All Fields] AND "vascular"[All Fields]) OR "vascular endothelium"[All Fields] OR ("vascular"[All Fields] AND "endothelium"[All Fields])) AND ("intercellular signaling peptides and proteins"[MeSH Terms] OR ("intercellular"[All Fields] AND "signaling"[All Fields] AND "peptides"[All Fields] AND "proteins"[All Fields]) OR "intercellular signaling peptides and proteins"[All Fields] OR ("growth"[All Fields] AND "factor"[All Fields]) OR "growth factor"[All Fields]))) AND (("coronaries"[All Fields] OR "heart"[MeSH Terms] OR "heart"[All Fields] OR "coronary"[All Fields]) AND ("collateral circulation"[MeSH Terms] OR ("collateral"[All Fields] AND "circulation"[All Fields]) OR "collateral circulation"[All Fields]))
A total of 3,249 articles were initially identified by assessment of titles and abstracts; of these, 1,975 articles remained after removing duplicates. The flowchart of study selection is shown in Figure 1.
Article inclusion and exclusion criteria
Inclusion criteria:
- The article must be a case control study or a cohort study including an English abstract.
- Patients in the case group had a clear coronary angiogram of at least one vessel with stenosis ≥50%. The control group are from healthy people.
- CCC was evaluated according to the results of coronary angiography and graded by Rentrop grade, which is a widely used method to evaluate the formation of collateral circulation.
- The results of VEGF measurement were obtained from blood rather than pericardial fluid or biopsies;
Exclusion criteria: articles on VEGF expression in blood cells by RT-PCR were excluded because the results were not comparable with plasma or serum VEGF. Literature reviews, articles involving exogenous administration of VEGF (including various VEGF-related gene therapies and application of various analogues), animal experiments, cellular experiments, and samples from sources other than blood. Studies not relevant to the topic of this article were identified by reading the abstract and title.
Data extraction and quality assessment
Two researchers independently assessed the quality of the included articles, using the Newcastle-Ottawa Scale (NOS) standards for assessing risk of bias. Articles with NOS scores <5 were removed after evaluation, and data were extracted for articles that passed the quality assessment. Extraction method: two researchers selected studies from the abstract and full-text articles, respectively, and performed quality assessment and data extraction. Data included in the study included author, country, race, year of publication, study design, sample size, circulating VEGF concentration (mean ± SD) in cases, circulating VEGF concentration (mean ± SD) in controls, age, sex, diabetes mellitus (DM), smoking history, history of hypertension, coronary heart disease, and family history. Both researchers thoroughly discussed the extracted data and reached a consensus. The source where the articles can be found was shown in Table S1.
Statistical analysis
Circulating VEGF was compared between controls and patients with CAD using standardized mean difference (SMD) and its 95% confidence interval (95% CI). Both these values were used to compare circulating VEGF concentrations between patients with good CCC and those with poor collateral circulation. A random-effects model was used to account for within-study and between-study heterogeneity. A heterogeneity index (I2) (range: 0–100%)—defined as the percentage of observed variability caused by heterogeneity rather than chance—was used to test for heterogeneity between studies. To identify potential sources of heterogeneity, we performed pre-defined subgroup analyses. Funnel plots were plotted to assess publication bias. Further, we performed a meta-regression analysis to assess the effect of confounding factors on heterogeneity. The above statistical analysis was completed using STATA software 13.0.
Results
The concentration of VEGF in patients with CAD is different from that in healthy people
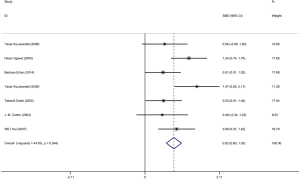
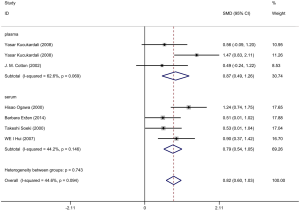
Six articles were included and information about each study is shown in Tables 1,2. Overall, 298 patients underwent coronary artery angiography (the degree of stenosis of at least one coronary artery indicated by coronary angiography was >50%), and 140 healthy control groups had enrolled in this pooled analysis. The VEGF concentration in patients with CAD was significantly higher than that in the control group (SMD: 0.82, 95% CI: 0.60–1.03). The heterogeneity was analyzed by meta-analysis (Figure 2). The results showed that the confounding factors influencing circulating VEGF did not affect the heterogeneity of the results. Funnel plot analysis of publication bias showed that the included articles were distributed symmetrically (Figure S1). Subgroup analysis showed that the concentration of VEGF in patients with coronary heart disease was significantly higher than that in the normal control group, and the heterogeneity was acceptable (Figure 3). In addition to this, a meta-regression was performed to analyze where the heterogeneity come from (Table S2).

Full table

Full table
VEGF and the establishment of collateral circulation in patients with severe CAD
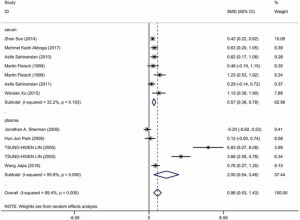
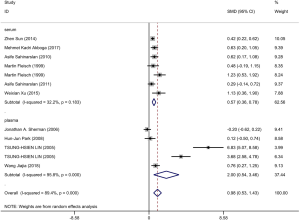
Ten articles were included and information about each study is shown in Tables 3,4. A total of 506 patients with good CCC confirmed by coronary angiography (Rentrop grade 2–3) and 633 patients with poor CCC (Rentrop grade 0–1) were included in the meta-analysis. The circulatory level of VEGF was higher (SMD: 0.98, 95% CI: 0.53–1.43) in the group with good collateral circulation than in the group with poor circulation. However, the heterogeneity was very significant (I2=89.4%, P<0.05). Next, subgroup analysis was conducted according to blood composition. Subgroup analysis showed that serum VEGF levels in patients with CAD with good collateral circulation was higher than in patients with poor collateral circulation, but the heterogeneity was not significant (Figure 4). Therefore, we speculated that blood composition may be one of the sources of heterogeneity. In addition, we conducted meta-regression analysis. Some studies have shown that the plasma VEGF level in diabetic patients with or without coronary vascular disease is significantly higher, and it is positively correlated with hyperglycemia (26). Alomari et al. compared the serum VEGF of four groups of cigarette smokers alone, hookah smokers alone, cigarette and hookah smokers, and non-smokers in an adolescent population, and found that serum VEGF in the non-smoking group was significantly higher than that in the other three groups, so smoking was one of the confounding factors affecting VEGF (27). Meta-regression analysis (Table S3) showed that smoking history was the source of heterogeneity. Other factors such as hypertension and diabetes could not explain the heterogeneity. We also found severe publication bias, which is presented in Figure S2.

Full table
Full table
Discussion
Early observations of fast-growing tumors have shown faster tumor tissue growth is associated with the increased growth of new blood vessels surrounding the tumor. Researchers have hypothesized that because malignant tumor cells require large quantities of oxygen and nutrients to sustain growth, they develop new blood vessels and trigger vascular network formation to acquire access to blood. Based on this hypothesis, researchers isolated VEGF, a cytokine that can promote tumor angiogenesis (28). As a growth factor, VEGF has important pro-angiogenic activity, acting primarily on vascular endothelial cells to trigger mitogen activation and anti-apoptotic effects, which can increase vascular permeability and promote cell migration (8). In severe CAD, such as CTO associated with the complete occlusion of the coronary artery, the pressure gradient on both sides of the potential collateral circulation increases, resulting in increased blood flow velocity and tangential fluid shear stress exerted on endothelial cells. These changes can trigger a series of cellular responses, including the altered expression of cell adhesion factors, monocyte aggregation, and the release of inflammatory cytokines such as VEGF. VEGF is involved in the process of arteriogenesis, which can promote the formation of collateral vessels to alleviate ischemia hypoxia (7,29). Therefore, VEGF can serve as a protective factor against coronary heart disease. Acute cardiovascular events in patients with coronary heart disease are often caused by the acute rupture of atherosclerotic plaques and severe coronary artery blockage, which can initiate a series of further complications. The infiltration of new blood vessels into atherosclerotic plaques plays an essential role in the process of plaque growth and the reduction of plaque stability. However, VEGF promotes capillary permeability, which is a risk factor for intraplaque hemorrhage (30,31). Under physiological conditions, VEGF-A and its receptors are not expressed in normal coronary arteries; however, in the presence of atherosclerotic lesions, VEGF-A expression increases in vascular endothelial cells, macrophages, and partially differentiated smooth muscle cells (32). Animal experiments have demonstrated that VEGF can be used as a marker of atherosclerosis (33). Therefore, VEGF is thought to play a dual role in coronary heart disease, but whether the circulatory levels of VEGF in patients with CAD increase or decrease remains unclear.
In this study, we performed a meta-analysis to explore whether differences could be detected in the VEGF levels in healthy controls compared with CAD patients and to determine whether VEGF could be used as a biomarker for CAD diagnosis. After searching the relevant literature and performing screening and quality evaluation, six articles were identified for inclusion in the final analysis. The meta-analysis showed that VEGF levels were higher in CAD patients than in healthy individuals, and the heterogeneity among the articles was found to be within the acceptable range, which further indicated the potential ability of VEGF to serve as a biomarker. In addition, 10 studies that discussed the effects of VEGF on collateral circulation were included in this analysis. The results of these studies showed that although the expression level of VEGF in CAD with good collateral circulation was higher than that in CAD with poor collateral circulation, significant heterogeneity existed. Therefore, we conducted subgroup analyses according to whether the biological samples consisted of plasma or serum. The subsequent meta-regression analysis revealed the influence of other confounding factors on heterogeneity (34), which impacted the final results (35). Experimental errors can be reduced by preparing serum and plasma samples simultaneously or through the use of anticoagulants (e.g., Pect Medium) containing prostaglandin E1 and theophylline, which can inhibit the activation and formation of artificial in vitro platelets during blood collection procedures (36). In addition, other factors that might influence the heterogeneity of the meta-analysis, such as a history of diabetes, smoking, hypertension, and family history of coronary heart disease, were also explored using meta-regression analysis, which indicating that smoking history was a confounding factor that influenced heterogeneity and that smoking was a primary risk factor for the development of atherosclerosis. Michaud et al. used human umbilical vein endothelial cells (HUVECs) and a mouse hind limb model to conduct in vitro and in vivo experiments, in which smoking conditions were simulated through the application of cigarette extracts to explore the effects of smoking on angiogenesis under hypoxic conditions. The results showed that cigarette extracts were able to inhibit the formation of capillary-like structures and the expression of VEGF protein in HUVECs in vitro. Cigarette extract-treated mice also displayed reduced capillary network density and slower blood flow velocity than untreated control mice. On the other hand, the impact of VEGF on the establishment of CCC is debatable. Previous study demonstrated that using VEGF to promote vessel growth obtained the modest result. Besides, VEGF receptor 2 which is identifiable on the endothelial surface and could hence be responsible for endothelial mitosis and inactivation of this receptor was shown to prevent the arteriogenesis in rats shows up-regulation when the mitosis activity of endothelial cell is over (37,38). Therefore, smoking appears to affect the expression of VEGF in vascular endothelial cells and inhibit compensatory neovascularization under hypoxic conditions. Previous study also indicated that smoking might inhibit VEGF by reducing the expression of HIF-1α, inhibiting tissue angiogenesis (12).
The primary limitation of the present study was that differences in VEGF levels could only be studied between CAD patients and healthy controls, and we were unable to analyze the diagnostic value of VEGF for CAD or explore which VEGF concentrations were associated with the maximum diagnostic efficiency. A diagnostic meta-analysis would be better able to reveal the diagnostic significance of VEGF. In addition, this meta-analysis only included CAD patients with diagnosis confirmed by coronary angiography. Coronary angiography remains the gold standard for CAD diagnosis; however, some CAD patients present with normal coronary angiography results, although this outcome is rare. Moreover, the current meta-analysis revealed that patients with CAD have higher levels of circulating VEGF than healthy controls, and we explored the influence on heterogeneity associated with various confounding factors that are known to confer additional cardiovascular risk, which improved the robustness of our analysis. The identification of this bio-marker has been associated with the early detection of cardiovascular risk, allowing doctors to take preventive action before CAD develops, which may have long-term benefits for patient health.
Conclusions
VEGF can be a potential biomarker to predict the occurrence of CAD. In addition, the concentration of circulating VEGF in patients with severe CAD is related to the degree of establishment of collateral circulation, and the level of VEGF in subjects with good collateral circulation is higher than in those with poor collateral circulation. Besides, future investigation on the potential of VEGF as a biomarker to help CADs diagnosis needs sensitivity and specificity test.
Acknowledgments
Funding: This work was supported by the National Science Foundation of China (grant number:51272287). Thanks for Pro. Yuanzhe Jin providing this idea, and Doc. Tian who made great endeavor for this study.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/jxym-21-3
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym-21-3). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation 2007;115:1285-95. [Crossref] [PubMed]
- Ogawa H, Suefuji H, Soejima H, et al. Increased Blood Vascular Endothelial Growth Factor Levels in Patients with Acute Myocardial Infarction. Cardiology 2000;93:93-9. [Crossref] [PubMed]
- Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol 2003;23:168-75. [Crossref] [PubMed]
- Cai W, Schaper W. Mechanisms of arteriogenesis. Acta Biochim Biophys Sin 2008;40:681-92. [Crossref] [PubMed]
- He Y, Luo Y, Tang S, et al. Critical function of Bmx/Etk in ischemia-mediated arteriogenesis and angiogenesis. J Clin Invest 2006;116:2344-55. [Crossref] [PubMed]
- Apte RS, Chen DS, Ferrara N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019;176:1248-64. [Crossref] [PubMed]
- Shen Y, Ding FH, Dai Y, et al. Reduced coronary collateralization in type 2 diabetic patients with chronic total occlusion. Cardiovasc Diabetol 2018;17:26. [Crossref] [PubMed]
- Melincovici CS, Boşca AB, Şuşman S, et al. Vascular endothelial growth factor (VEGF) - key factor in normal and pathological angiogenesis. Rom J Morphol Embryol 2018;59:455-67. [PubMed]
- Celletti FL, Waugh JM, Amabile PG, et al. Vascular endothelial growth factor enhances atherosclerotic plaque progression. Nat Med 2001;7:425. [Crossref] [PubMed]
- Wahl A, Billinger M, Fleisch M, et al. Pathophysiology of coronary collateral circulation in the human. Schweiz Med Wochenschr 1998;128:1527-37. [PubMed]
- Sun Z, Shen Y, Lu L, et al. Increased serum level of soluble vascular endothelial growth factor receptor-1 is associated with poor coronary collateralization in patients with stable coronary artery disease. Circ J 2014;78:1191-6. [Crossref] [PubMed]
- Kucukardali Y, Aydogdu S, Ozmen N, et al. The relationship between severity of coronary artery disease and plasma level of vascular endothelial growth factor. Cardiovasc Revasc Med 2008;9:66-70. [Crossref] [PubMed]
- Soeki T, Tamura Y, Shinohara H, et al. Role of circulating vascular endothelial growth factor and hepatocyte growth factor in patients with coronary artery disease. Heart Vessels 2000;15:105-11. [Crossref] [PubMed]
- Cotton JM, Mathur A, Hong Y, et al. Acute rise of circulating vascular endothelial growth factor-A in patients with coronary artery disease following cardiothoracic surgery. Eur Heart J 2002;23:953-9. [Crossref] [PubMed]
- Wei H, Zeng Qiutang. Measurement of serum vascular endothelial growth factor and its clinical significance in percutaneous coronary intervention and diagnosing coronary Heart disease. J Clin Cardiol 2007;23:829-31.
- ErŽen B. Šilar M, Šabovič M. Stable phase post-MI patients have elevated VEGF levels correlated with inflammation markers, but not with atherosclerotic burden. BMC Cardiovasc Disord 2014;14:166. [Crossref] [PubMed]
- Akboğa MK, Taçoy G, Yılmaz DC, et al. As cardioprotective and angiogenic biomarker, can ghrelin predict coronary collateral development and severity of coronary atherosclerosis? Turk Kardiyol Dern Ars 2017;45:316-23. [PubMed]
- Sherman JA, Hall A, Malenka DJ, et al. Humoral and Cellular Factors Responsible for Coronary Collateral Formation. Am J Cardiol 2006;98:1194-7. [Crossref] [PubMed]
- Sahinarslan A, Kocaman S, Topal S, et al. Relation between serum monocyte chemoattract-ant protein-1 and coronary collateral development. Coron Artery Dis 2010;21:455-9. [Crossref] [PubMed]
- Park HJ, Chang K, Chan SP, et al. Coronary collaterals: The role of MCP-1 during the early phase of acute myocardial infarction. Int J Cardiol 2008;130:409-13. [Crossref] [PubMed]
- Lin TH, Yen HW, Voon WC, et al. Vascular endothelial growth factor in coronary sinus: Evidence for its association with coronary collaterals. Scand Cardiovasc J 2005;39:353-7. [Crossref] [PubMed]
- Fleisch M, Billinger M, Eberli FR, et al. Physiologically Assessed Coronary Collateral Flow and Intracoronary Growth Factor Concentrations in Patients With 1- to 3-Vessel Coronary Artery Disease. Circulation 1999;100:1945. [Crossref] [PubMed]
- Sahinarslan A, Yalcin R, Kocaman SA, et al. The Relationship of Serum Erythropoietin Level With Coronary Collateral Grade. Can J Cardiol 2011;27:589-95. [Crossref] [PubMed]
- Xu W, Guo Z, Mi L, et al. Serum erythropoietin: a useful biomarker for coronary collateral development and potential target for therapeutic angiogenesis among the patients with coronary chronic total occlusion. Biomarkers 2013;18:343-8. [Crossref] [PubMed]
- Wang J, Guo R, He F, et al. Study on the correlation between plasma ESM-1 level and for-mation of coronary collateral circulation in patients with chronic coronary occlusion. Henan Med Res 2018;27:1931-5.
- Lim HS, Lip GY, Blann AD. Angiopoietin-1 and angiopoietin-2 in diabetes mellitus: relationship to VEGF, glycaemic control, endothelial damage/dysfunction and atherosclerosis. Atherosclerosis 2005;180:113-8. [Crossref] [PubMed]
- Alomari MA, Al-Sheyab N, Khabour O, et al. Serum VEGF Level Is Different in Adolescents Smoking Waterpipe versus Cigarettes: The Irbid TRY. Biomolecules 2018;8:102. [Crossref] [PubMed]
- Lugano R, Ramachandran M, Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci 2020;77:1745-70. [Crossref] [PubMed]
- Schaper W, Buschmann I. Arteriogenesis, the good and bad of it. Eur Heart J 1999;20:1297-9. [Crossref] [PubMed]
- Chen CH, Walterscheid JP. Plaque Angiogenesis Versus Compensatory Arteriogenesis in Atherosclerosis. Circ Res 2006;99:787-9. [Crossref] [PubMed]
- Braile M, Marcella S, Cristinziano L, et al. VEGF-A in Cardiomyocytes and Heart Diseases. Int J Mol Sci 2020;21:5294. [Crossref] [PubMed]
- Ylä-Herttuala S, Rissanen TT, Vajanto I, et al. Vascular endothelial growth factors: biology and current status of clinical applications in cardiovascular medicine. J Am Coll Cardiol 2007;49:1015-26. [Crossref] [PubMed]
- Yu ZM, Deng XT, Qi RM, et al. Mechanism of Chronic Stress-induced Reduced Atherosclerotic Medial Area and Increased Plaque Instability in Rabbit Models of Chronic Stress. Chin Med J (Engl) 2018;131:161. [Crossref] [PubMed]
- Gunsilius E, Petzer A, Stockhammer G, et al. Thrombocytes Are the Major Source for Soluble Vascular Endothelial Growth Factor in Peripheral Blood. Oncology 2000;58:169-74. [Crossref] [PubMed]
- Schlingemann RO, Van Noorden CJF, Diekman MJM, et al. VEGF Levels in Plasma in Relation to Platelet Activation, Glycemic Control, and Microvascular Complications in Type 1 Diabetes. Diabetes Care 2013;36:1629-34. [Crossref] [PubMed]
- Levi M, Biemond BJ, Van Zonneveld AJ, et al. Inhibition of plasminogen activator inhibitor-1 activity results in promotion of endogenous thrombolysis and inhibition of thrombus extension in models of experimental thrombosis. Circulation 1992;85:305. [Crossref] [PubMed]
- Heil M, Schaper W. Insights into pathways of arteriogenesis. Curr Pharm Biotechnol 2007;8:35-42. [Crossref] [PubMed]
- Cai W, Schaper W. Mechanisms of arteriogenesis. Acta Biochim Biophys Sin (Shanghai) 2008;40:681-92. [Crossref] [PubMed]
Cite this article as: Wang T, Tian J, Han S, Jin Y. Correlation between circulating vascular endothelial growth factor levels and coronary artery disease and its influence on the establishment of coronary collateral circulation: a systematic review and meta-analysis. J Xiangya Med 2021;6:14.



