The predictive effect of shock index on mortality in patients with acute heart failure
Highlight box
Key findings
• The higher the derivatives of the shock index (SI), the lower the in-hospital survival rate.
What is known and what is new?
• The SI has shown predictive relevance in myocardial infarction, pulmonary embolism even in individuals with normal blood pressure and heart rate values.
• SI and age-adjusted SI (SI × age) index value in the context of the acute heart failure (AHF) is unknown.
What is the implication, and what should change now?
• We can estimate in-hospital mortality using SI and SI × age index in patients with AHF.
Introduction
In all diseases, it is a clinical challenge to use basic admission variables to determine which patients are more likely to be a hospitalization by comorbidities or to die before discharge. Acute heart failure (AHF), is a common cause of hospitalization globally in coronary care units (CCUs) (1). AHF carries a risk of in-hospital mortality ranging from 5% and 16%, primarily in the elderly, based on the populations investigated (2,3). Using the parameters available since the patient’s entrance, it is difficult to determine which individual is most likely to die.
Allgower and Burri created the shock index (SI) notion in 1967 and explored its application in the situation of hypovolemic shock (4). Subsequently, experimental and clinical research revealed an adverse correlation between SI and physiological markers including ventricular work index, cardiac index, mean arterial pressure, and stroke volume (5). The SI, derived from the admission values of heart rate (HR) and systolic blood pressure (SBP), has shown predictive relevance in other contexts, including myocardial infarction, pulmonary embolism, severe sepsis, and trauma, even in individuals with normal BP and HR values (6-9). However, its value in the context of the AHF is unknown. Similarly, it has been described that adjusting the SI to the patient’s age offers better prognostic data (10).
The study aimed to determine the predictive value of SI and age-adjusted SI (SI × age) for in-hospital mortality in patients admitted to the CCU with AHF. We present this article in accordance with the STARD reporting checklist (available at https://jxym.amegroups.com/article/view/10.21037/jxym-23-20/rc).
Methods
Study design and subject
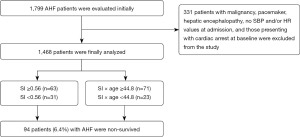
This study was carried out as a retrospective case-control study between 2014 and 2022. Patients admitted to this study were >18 years of age and were admitted with AHF to the CCU. In total, 1,799 patients were consecutively included in the study. A total of 331 patients with malignancy, pacemaker, hepatic encephalopathy, no SBP and/or HR values at admission, and those presenting with cardiac arrest at baseline were excluded from the study because their indexes could be calculated incorrectly. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of Health Sciences University, Gazi Yaşargil Training and Research Hospital (No. 2023-301) and informed consent was taken from all the patients.
Study protocol
The patients’ social demographic and clinical characteristics were recorded. Blood tests were conducted routinely on all patients. As part of the standard care, patients were monitored from the point of their admission to the CCU until their discharge. Vital signs were obtained with a bedside monitor (GE Healthcare B40 V3 Patient Monitor, Cary, NC, USA). SBP and HR were measured and recorded on admission to the CCU. Simultaneous blood pressure was measured while intravenous access was established. The first measurement was not accepted and the average of the second and third measurements was used for analysis (11). SI and SI adjusted for age were computed. The predictive value of both indices for mortality was analyzed (Figure 1). Youden’s index was applied to calculate the optimal SI and SI × age cut-off for estimating mortality. This value was utilized to stratify the research population into two groups: non-survivors and survivors. Blood urea nitrogen (BUN) >43 mg/dL, creatine >2.75 mg/dL, hemoglobin (Hb) <10 g/dL, and SBP <115 mmHg values, which are the best predictors of in-hospital mortality in AHF, were evaluated and regressed along with SI and SI × age values (12).
Definitions
AHF refers to the sudden or gradual emergence of symptoms or indications of HF that are severe enough to urge the patient to seek immediate medical attention, resulting in an unplanned hospital admission or visit to the emergency room (13).
The SI is a quick and useful indicator that can alert clinicians of potential causes of shock including myocardial infarction, pulmonary embolism, hemorrhage, and sepsis (14). The SI was computed by utilizing the formula: HR/SBP and the SI adjusted for age was computed utilizing the formula SI × age.
Based on previous studies, BUN >43 mg/dL, creatine >2.75 mg/dL, Hb <10 g/dL, and SBP <115 mmHg values were accepted as the best predictors of in-hospital mortality in AHF, and SI and SI × age values were evaluated and regressed.
Statistical analysis
The IBM SPSS 24.0 package software (Armonk, NY, USA) was applied for the analysis. Initial continuous variables are shown as median (interquartile range). Categorical variables were represented with frequencies and percentages. The Chi-square test or Fisher’s test was utilized for categorical variables. Student’s t-test or Mann-Whitney U test was utilized to compare continuous variables, as appropriate. The receiving operating characteristic (ROC) curve for SI and for SI × age, as well as the Youden index, were constructed to determine the optimal sensitivity and specificity values for predicting in-hospital mortality. The independent determinants of in-hospital mortality were established using simple and multivariate logistic regression. The threshold of statistical significance for the data was set at P<0.05.
Results
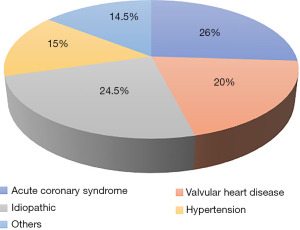
A total of 1,468 patients, comprising 53.7% males were included. The average age of the non-survivor group was 85 (79–91) years and 80 (73–86) years for survivor group. When their distribution according to comorbidities was examined, 80.6% had hypertension (HT), 14.0% were smoking, 26.9% had diabetes mellitus (DM), 22.8% had dyslipidemia (DL), 12.9% had thyroid dysfunction, 22.9% had history of ischemic heart disease (IHD), 51.0% had chronic heart failure (HF), 53.0% had left ventricular ejection fraction (LVEF) <40% and 9.0% had a stroke (Table 1). There was a history of beta-blocker use in 32.0%, angiotensin converting enzyme inhibitor (ACEI)/angiotensin receptor blocker (ARB)/angiotensin receptor-neprilysin inhibitor (ARNI) use in 18.3%, and furosemide in 23.0%. There were no statistical differences among the groups including comorbidities and drug use [P= non-statistically significant (NS)]. The etiology of AHF was coronary in 26%, valvular in 20%, hypertensive in 15%, idiopathic in 24.5%, and 14.5% due to other causes (Figure 2); 13.3% of the patients required inotropes, 51.2% invasive ventilation, and in-hospital mortality was 6.4%. There was no significant difference in mortality between non-survivors with LVEF <40 or LVEF ≥40, positive troponin or natriuretic peptide, and invasive or noninvasive mechanical ventilation (P>0.05). In the survivor group, the median SI was 0.6 (0.5–0.75), and the median SI × age was 46 (38–58). In the non-survivor group, the median SI was 0.62 (0.55–0.81) and the median SI × age was 53 (44–66).
Table 1
| Parameters | Non-survivor (n=94) | Survivor (n=1,374) | P value |
|---|---|---|---|
| Age (years) | 85 [79–91] | 80 [73–86] | <0.01 |
| Male | 46 (48.9) | 742 (54.0) | NS |
| History of stroke | 6 (6.3) | 124 (9.0) | NS |
| History of IHD | 16 (17.0) | 315 (22.9) | NS |
| History of HF | 52 (55.3) | 701 (51.0) | NS |
| Hypertension | 76 (80.9) | 1,108 (80.6) | NS |
| Diabetes mellitus | 29 (30.9) | 370 (26.9) | NS |
| Thyroid dysfunction | 14 (14.9) | 177 (12.9) | NS |
| DL | 21 (22.3) | 313 (22.8) | NS |
| Smoking | 13 (13.8) | 192 (14.0) | NS |
| BUN >43 mg/dL | 70 (74.5) | 934 (68.0) | <0.01 |
| Creatinine >2.75 mg/dL | 15 (16.0) | 97 (7.1) | <0.01 |
| Anemia (Hb <10 g/dL) | 31 (33.0) | 237 (17.2) | <0.01 |
| SBP <115 mmHg | 21 (22.3) | 269 (19.6) | 0.02 |
| HR >100 beats/minute | 28 (29.8) | 316 (23.0) | NS |
| Troponin positivity | 47 (50.0) | 742 (54.0) | NS |
| Natriuretic peptide positivity | 52 (55.3) | 701 (51.0) | NS |
| Invasive mechanical ventilation | 51 (54.2) | 703 (51.2) | NS |
| LVEF <40% | 47 (50.0) | 728 (53.0) | NS |
| Inotropes support | 17 (18.1) | 183 (13.3) | NS |
| Beta-blockers use | 32 (34.0) | 439 (32.0) | NS |
| ACEI/ARB/ARNI use | 14 (14.9) | 252 (18.3) | NS |
| Furosemide use | 19 (20.2) | 315 (23.0) | NS |
| Median SI | 0.62 [0.55–0.81] | 0.6 [0.5–0.75] | <0.01 |
| Median SI × age | 53 [44–66] | 46 [38–58] | <0.01 |
Data are expressed as number (percentage), or median [interquartile range] as appropriate. NS, non-statistically significant; IHD, ischemic heart disease; HF, heart failure; DL, dyslipidemia; BUN, blood urea nitrogen; Hb, hemoglobin; SBP, systolic blood pressure; HR, heart rate; LVEF, left ventricular ejection fraction; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor-neprilysin inhibitor; SI, shock index.
The ROC curves of both indices for in-hospital mortality are shown in Figure 3. The area under the curve (AUC) was 0.592 [95% confidence interval (CI): 0.536–0.648, P=0.003] for the SI and 0.628 (95% CI: 0.572–0.683, P<0.0001) for the SI × age. In accordance with the Youden index, the optimal SI value to predict mortality was 0.56 with a specificity of 46% and a sensitivity of 70%, providing a negative predictive value (NPV) of 96% and a positive predictive value (PPV) of 8%. Sixty-three patients (67%) with high SI died during follow-up compared with 31 patients (33%) with low SI. The optimal SI × age value to predict mortality was 44.8, with a specificity of 48% and a sensitivity of 76%, providing an NPV of 97% and a PPV of 7%. Seventy-one patients (76%) with high SI × age died during follow-up compared with 24 patients (24%) with low SI × age. In order to increase specificity, different cut-off values for both SI and SI × age were considered. Table 2 shows each index’s prevalence, sensitivity, specificity, NPV, and PPV for mortality cut-off points. It is observed that with the increase in the value of the indices, the specificity rise, and the PPV somewhat, and although the sensitivity decreases, the NPV also remains at high values (94–95%). There was a correlation between in-hospital mortality and differing SI and SI × age values.

Table 2
| Predictor | Prevalence (%) | Sensitivity (%) | Specificity (%) | NPV (%) | PPV (%) |
|---|---|---|---|---|---|
| SI | |||||
| ≥0.56 | 67 | 70 | 46 | 96 | 8 |
| ≥0.7 | 33 | 42 | 68 | 95 | 8 |
| ≥0.8 | 20 | 27 | 81 | 94 | 8 |
| SI × age | |||||
| ≥44.8 | 76 | 76 | 48 | 97 | 7 |
| ≥50 | 46 | 56 | 62 | 95 | 8 |
| ≥60 | 26 | 39 | 74 | 95 | 10 |
SI, shock index; NPV, negative predictive value; PPV, positive predictive value.
In the multivariate analysis that included age, Hb <10 g/dL, SBP <115 mmHg, creatinine >2.75 mg/dL, BUN >43 mg/dL, and SI greater than or equal to 0.56, only age [odds ratio (OR) =1.04; 95% CI: 1.00–1.06; P<0.01], anemia (OR =2.43; 95% CI: 1.45–4.09, P<0.01) and BUN >43 mg/dL (OR =2.36; 95% CI: 1.17–4.78; P<0.01) maintained their predictive value (model 1, Table 3). But in the multivariate model 2 where the SI × age ≥44.8 was evaluated together with the other variables (except age), this was an independent predictor (OR =2.38; 95% CI: 1.35–4.18; P<0.01) as well as anemia (OR =2.36; 95% CI: 1.45–4.09; P<0.01) and BUN >43 mg/dL (OR =2.35; 95% CI: 1.22–4.78; P<0.01). The power of SI × age to predict mortality was 2.39 times greater than other independent predictors.
Table 3
| Predictor | OR | 95% CI | P value |
|---|---|---|---|
| Model 1 | |||
| Age | 1.04 | 1.00–1.06 | <0.01 |
| SBP <115 mmHg | 1.21 | 0.58–2.18 | 0.74 |
| BUN >43 mg/dL | 2.36 | 1.17–4.78 | <0.01 |
| Creatine >2.75 mg/dL | 1.73 | 0.86–3.41 | 0.11 |
| Hb <10 g/dL | 2.43 | 1.45–4.09 | <0.01 |
| SI ≥0.56 | 1.54 | 0.89–2.61 | 0.12 |
| Model 2 | |||
| SBP <115 mmHg | 1.00 | 0.52–1.79 | 0.98 |
| BUN >43 mg/dL | 2.35 | 1.22–4.78 | <0.01 |
| Creatinine >2.75 mg/dL | 1.65 | 0.86–3.19 | 0.12 |
| Hb <10 g/dL | 2.36 | 1.45–4.09 | <0.01 |
| SI × age ≥44.8 | 2.38 | 1.35–4.18 | <0.01 |
OR, odds ratio; CI, confidence interval; SBP, systolic blood pressure; BUN, blood urea nitrogen; Hb, hemoglobin; SI, shock index.
Discussion
We identified the persistent prognostic significance of admission SI by demonstrating that considerably poorer outcomes, including death, are associated with greater SI values. This relationship seems linear; each 0.1 increment in SI over a SI value of 0.6 appears to be a significant predictive factor. Similarly, we found every 10 increases in the SI × age value above 50 to be an important value for mortality.
SI reflects the integration of the cardiovascular system and the central nervous system, for which reason high values indicate hyperactivity of the autonomic nervous system, which could contribute to myocardial damage and/or lethal arrhythmias. Its normal value in healthy individuals is between 0.5 and 0.7, and its predictive usefulness is dependent on the cut-off values employed since they provide varying sensitivity and specificity for events (15). In most studies, a value >0.7 is taken as the cut-off point (16). Higher values are associated with higher mortality, and its highest specificity occurs in values >0.9. Values close to 1 are indicative of worsening hemodynamic status and shock (17).
In our research, SI 0.56 and SI × age 44.8 exhibited the highest sensitivity and specificity for the Youden index. Both indices, at these levels, represent a very high NPV for mortality; hence, values less than 0.56 or 44.8 would indicate positive clinical progression. The higher the value of the indices, the higher the mortality rate.
Some authors argue that physiological changes or the usage of drugs such as beta-blockers or calcium channel blockers result in decreased HR in the elderly (18). These drugs can affect HR in response to the drop in minute volume, which weakens the prognostic power of SI (19). It is postulated that using SI × age achieves greater sensitivity and specificity in this subgroup (20). In recent studies, the SI × age has predicted mortality better than the classic SI in emergency room patients (21,22). In pathologies such as pulmonary embolism, its significant NPV (>95%) in prognostic classification is comparable to other markers, such as troponin (23). The indices are fundamentally used to ensure a good prognosis when they are low, due to their very high NPV. When they are high, they are less effective for determining risk.
The bibliography on the value of the SI and the SI × age in the context of decompensated heart failure (DHF) is scarce and even presents some contradictory results. Liu et al. Found that the SI index was not associated with mortality in 22,161 emergency patients admitted to the emergency department (24). Likewise, Koch et al. Stated that SI was not associated with mortality in acute hypovolemia’s early and late stages (25). Contrary to these studies, Cannon et al. observed that SI was correlated with mortality in traumatically injured patients (26). In El-Menyar et al. study, with 5,005 patients, both SI and SI × age were independent predictors of events (27). On the other hand, Pourafkari et al. stated that SI had no predictive significance in DHF patients, but they postulated that SI × age could play a role (28). In the research conducted by El-Menyar et al., the median SI was determined to be 0.74, and the SI threshold for distinguishing mortality was 0.9 (median age 59 years). However, when examining the association between SI and age, the median value of SI in patients older than 75 years (such as those of Pourafkari and ours) was approximate to those of our research group (0.68 vs. 0.61). In this research, both indices were mortality predictors, however, the SI × age index ensured better prognostic distinction. Our patients were much older than those in the El-Menyar study, which could explain the difference in the use of the indexes.
Likewise, hypotension on admittance is an independent predictor of complications and mortality both in the hospital phase and in the long term in AHF, but it loses predictive significance compared to SI, as we found in our research (29). The SI offers additional prognostic information to that of the individual vital variables that compose it, even when these are within normal ranges, for this reason, its prognostic significance was evaluated in a variety of clinical situations with positive outcomes (30).
Renal failure and anemia on admission maintained their prognostic value for higher mortality in our model together with the SI × age. In Scicchitano et al. study, the rate of anemia was found to be higher in acute HF. Anemia was determined as a marker indicating the severity of the disease in patients who developed congestion, but was not an independent risk factor for mortality (31). The ADHERE registry comprised roughly 33,000 individuals with a mean age of 71.5 years (about 9 years younger than ours), a larger proportion of diabetes (42% vs. 27%), and chronic renal failure (28% vs. 21%) than our work (32). This research determined the predictive usefulness of a branched algorithm that comprised a BUN value >43 mg/dL, systolic BP <115 mmHg, and creatinine >2.75 mg/dL, thus discriminated populations with different in-hospital mortality from 9% to 21%. Renal failure is a widespread disease in the elderly, although its prevalence could vary significantly based on the criteria used, calculation of glomerular filtration by formulae, or significance level above the reference values (33,34). In another study, BNP >44 pg/mL, BUN >1.67 mmol/L, PaO2 ≤69.7 mmHg and phase angle ≤4.9° were found to be significant in predicting long-term mortality in patients with acute decompensated heart failure (35).
Limitations
This study is a retrospective analysis, and although it is of important dimensions, we cannot rule out findings defined by chance. In any case, there are no studies that have tested it prospectively. SI has a range of values, and no standardized definition of an aberrant SI has been created. Age is the most significant factor in determining the prognosis of patients with comorbidities; therefore, variations in its prevalence will affect the relative importance of the prognostic factors. In our study, which consisted primarily of individuals over the age of 80, the behavior of HR and BP likely demonstrates the substantial involvement of neurovegetative dysautonomia, which determines more lability on admission and even less response to established vasoactive medicines. Although it includes a population of elderly patients with AHF, the Charlson Comorbidity Index and CHA2DS2-Vasc Risk Score have not been specifically tested (36,37).
Conclusions
It is important to highlight that the populations included in the registries are heterogeneous in terms of their age conditions, and pathological backgrounds, which can influence the outcomes of the investigations. We emphasize that simple variables collected at the CCU admission of the patients make it possible to calculate the SI × age, a highly valuable parameter in the prediction of mortality in AHF patients, and that ensures extra information to the standard prognostic indicators.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jxym.amegroups.com/article/view/10.21037/jxym-23-20/rc
Data Sharing Statement: Available at https://jxym.amegroups.com/article/view/10.21037/jxym-23-20/dss
Peer Review File: Available at https://jxym.amegroups.com/article/view/10.21037/jxym-23-20/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jxym.amegroups.com/article/view/10.21037/jxym-23-20/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of Health Sciences University, Gazi Yaşargil Training and Research Hospital (No. 2023-301) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Delgado B, Novo A, Lopes I, et al. The effects of early rehabilitation on functional exercise tolerance in decompensated heart failure patients: Results of a multicenter randomized controlled trial (ERIC-HF study). Clin Rehabil 2022;36:813-21. [Crossref] [PubMed]
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017;70:776-803. [Crossref] [PubMed]
- Fairman E, Mauro V, Charask A, et al. Insuficiencia cardíaca descompensada ¿De qué estamos hablando? Rev Argent Cardiol 2018;86:359-62.
- Doğanay F, Elkonca F, Seyhan AU, et al. Shock index as a predictor of mortality among the Covid-19 patients. Am J Emerg Med 2021;40:106-9. Erratum in: Am J Emerg Med 2021;43:293. [Crossref] [PubMed]
- Wang G, Wang R, Liu L, et al. Comparison of shock index-based risk indices for predicting in-hospital outcomes in patients with ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention. J Int Med Res 2021;49:3000605211000506. [Crossref] [PubMed]
- Huang B, Yang Y, Zhu J, et al. Usefulness of the admission shock index for predicting short-term outcomes in patients with ST-segment elevation myocardial infarction. Am J Cardiol 2014;114:1315-21. [Crossref] [PubMed]
- Gupta S, Alam A. Shock index is better than conventional vital signs for assessing higher level of care and mortality in severe sepsis or shock. Am J Emerg Med 2021;46:545-9. [Crossref] [PubMed]
- Ozsu S, Erbay M, Durmuş ZG, et al. Classification of high-risk with cardiac troponin and shock index in normotensive patients with pulmonary embolism. J Thromb Thrombolysis 2017;43:179-83. [Crossref] [PubMed]
- Vang M, Østberg M, Steinmetz J, et al. Shock index as a predictor for mortality in trauma patients: a systematic review and meta-analysis. Eur J Trauma Emerg Surg 2022;48:2559-66. [Crossref] [PubMed]
- Yu T, Tian C, Song J, et al. Age Shock Index is Superior to Shock Index and Modified Shock Index for Predicting Long-Term Prognosis in Acute Myocardial Infarction. Shock 2017;48:545-50. [Crossref] [PubMed]
- Beaney T, Schutte AE, Stergiou GS, et al. May Measurement Month 2019: The Global Blood Pressure Screening Campaign of the International Society of Hypertension. Hypertension 2020;76:333-41. [Crossref] [PubMed]
- Fonarow GC, Adams KF Jr, Abraham WT, et al. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA 2005;293:572-80. [Crossref] [PubMed]
- McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599-726. [Crossref] [PubMed]
- Montoya KF, Charry JD, Calle-Toro JS, et al. Shock index as a mortality predictor in patients with acute polytrauma. Journal of Acute Disease 2015;4:202-4. [Crossref]
- Bondariyan N, Vakhshoori M, Sadeghpour N, et al. Prognostic Value of Shock Index, Modified Shock Index, and Age-Adjusted Derivatives in Prediction of In-Hospital Mortality in Patients with Acute Decompensated Heart Failure: Persian Registry of Cardiovascular Disease/ Heart Failure Study. Anatol J Cardiol 2022;26:210-7. [Crossref] [PubMed]
- Pramudyo M, Putra ICS, Kamarullah W, et al. Elevated shock index and modified shock index are associated with mortality and major adverse cardiac events in patients with acute myocardial infarction: A systematic review and meta-analysis. F1000Research 2022;11:926. [Crossref]
- Berger T, Green J, Horeczko T, et al. Shock index and early recognition of sepsis in the emergency department: pilot study. West J Emerg Med 2013;14:168-74. [Crossref] [PubMed]
- Kristensen AK, Holler JG, Hallas J, et al. Is Shock Index a Valid Predictor of Mortality in Emergency Department Patients With Hypertension, Diabetes, High Age, or Receipt of β- or Calcium Channel Blockers? Ann Emerg Med 2016;67:106-13.e6. [Crossref] [PubMed]
- Oh GC, An S, Lee HY, et al. Modified reverse shock index predicts early outcomes of heart failure with reduced ejection fraction. ESC Heart Fail 2022;9:3232-40. [Crossref] [PubMed]
- Jentzer JC, van Diepen S, Barsness GW, et al. Cardiogenic Shock Classification to Predict Mortality in the Cardiac Intensive Care Unit. J Am Coll Cardiol 2019;74:2117-28. [Crossref] [PubMed]
- Rappaport LD, Deakyne S, Carcillo JA, et al. Age- and sex-specific normal values for shock index in National Health and Nutrition Examination Survey 1999-2008 for ages 8 years and older. Am J Emerg Med 2013;31:838-42. [Crossref] [PubMed]
- Tzadok B, Soffer S, Shapira S, et al. Shock index in the acute care geriatric patient and its correlation to the need of a life saving intervention. Int J Gerontol Geriatr Res 2019;2:001-004.
- Torabi M, Mirafzal A, Rastegari A, et al. Association of triage time Shock Index, Modified Shock Index, and Age Shock Index with mortality in Emergency Severity Index level 2 patients. Am J Emerg Med 2016;34:63-8. [Crossref] [PubMed]
- Liu YC, Liu JH, Fang ZA, et al. Modified shock index and mortality rate of emergency patients. World J Emerg Med 2012;3:114-7. [Crossref] [PubMed]
- Koch E, Lovett S, Nghiem T, et al. Shock index in the emergency department: utility and limitations. Open Access Emerg Med 2019;11:179-99. [Crossref] [PubMed]
- Cannon CM, Braxton CC, Kling-Smith M, et al. Utility of the shock index in predicting mortality in traumatically injured patients. J Trauma 2009;67:1426-30. [Crossref] [PubMed]
- El-Menyar A, Sulaiman K, Almahmeed W, et al. Shock Index in Patients Presenting With Acute Heart Failure: A Multicenter Multinational Observational Study. Angiology 2019;70:938-46. [Crossref] [PubMed]
- Pourafkari L, Wang CK, Schwartz M, et al. Does shock index provide prognostic information in acute heart failure? Int J Cardiol 2016;215:140-2. [Crossref] [PubMed]
- Gheorghiade M, Abraham WT, Albert NM, et al. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA 2006;296:2217-26. [Crossref] [PubMed]
- Zampieri FG, Colombari F. Use of shock index as a prognostic marker in patients with normal heart rate and blood pressure at ICU admission. Intensive Care Med Exp 2015;3:A596. [Crossref]
- Scicchitano P, Iacoviello M, Massari A, et al. Anaemia and Congestion in Heart Failure: Correlations and Prognostic Role. Biomedicines 2023;11:972. [Crossref] [PubMed]
- Adams KF Jr, Fonarow GC, Emerman CL, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J 2005;149:209-16. [Crossref] [PubMed]
- Yusipov I, Kondakova E, Kalyakulina A, et al. Accelerated epigenetic aging and inflammatory/immunological profile (ipAGE) in patients with chronic kidney disease. Geroscience 2022;44:817-34. [Crossref] [PubMed]
- da Silva Selistre L, Rech DL, de Souza V, et al. Diagnostic Performance of Creatinine-Based Equations for Estimating Glomerular Filtration Rate in Adults 65 Years and Older. JAMA Intern Med 2019;179:796-804. [Crossref] [PubMed]
- Massari F, Scicchitano P, Iacoviello M, et al. Multiparametric approach to congestion for predicting long-term survival in heart failure. J Cardiol 2020;75:47-52. [Crossref] [PubMed]
- Formiga F, Moreno-Gonzalez R, Chivite D, et al. High comorbidity, measured by the Charlson Comorbidity Index, associates with higher 1-year mortality risks in elderly patients experiencing a first acute heart failure hospitalization. Aging Clin Exp Res 2018;30:927-33. [Crossref] [PubMed]
- Chen YL, Cheng CL, Huang JL, et al. Mortality prediction using CHADS2/CHA2DS2-VASc/R2CHADS2 scores in systolic heart failure patients with or without atrial fibrillation. Medicine (Baltimore) 2017;96:e8338. [Crossref] [PubMed]
Cite this article as: Günlü S, Kayan F, Karahan MZ. The predictive effect of shock index on mortality in patients with acute heart failure. J Xiangya Med 2023;8:5.