Aetiologies and correlates of secondary mitral regurgitation in patients with dilated cardiopathy in Cameroon: a cross-sectional study
Introduction
Dilated cardiopathy of non-valvular and non-congenital origin is frequent worldwide, and is often associated with secondary or functional mitral valve regurgitation (1-3). The prevalence of MR in the general population is not well characterized, and it is associated with a poor prognosis especially in those more than seventy years old with chronic heart failure (4). MR has been shown to have a direct correlation with mortality. Mitral valvuloplasty is the mainstay of treatment for sever MR, and has been shown to improve symptoms (5). However, the post-surgical mortality is non-negligible and the relapse rate is high (5). Percutaneous mitral clip has shown better outcome than mitral valvuloplasty (6). This suggests that reduction of MR should strongly be considered as a therapeutic target in patients with heart disease.
Data on the causes and correlates of secondary MR in sub-Saharan Africa (SSA) are rare, as they are often considered as satellite to heart failure. This work aimed at studying the clinical and echocardiographic aspects, and the aetiologies of secondary MR in patients with dilated cardiopathy of non-valvular and non-congenital cause in a low-income setting in SSA.
Methods
We carried out this work in accordance with the declarations of Helsinki (7). Ethical clearance was obtained from the ethical committee of the Faculty of Medicine and Biomedical Sciences, University of Yaounde 1. We report this work in accordance with the Standard for Reporting Observational Studies (STROBE) checklist (8).
Study design and setting
Between October 2015 and May 2016, we carried out a cross-sectional study in echocardiography laboratories of three tertiary Hospitals in the city of Yaounde, the political capital of Cameroon, sub-Saharan Africa. The catchment population of these hospitals is about two million inhabitants, with a total of ten cardiologists. These Hospitals serve as the main teaching hospitals of the University of Yaounde 1.
Participants
Participants were consenting adult patients of both sex, aged ≥18 years, who had an echocardiographic diagnosis of non-valvular and non-congenital dilated cardiopathy with or without MR. We excluded patients with pregnancy, anemia, and non-consent.
Variables
Consenting patients were interviewed and examined. We obtained the following clinical information. Socio-demography (age and sex), symptoms of heart disease (dyspnea, orthopnea, paroxysmal nocturnal dyspnea, cough, chest pain, palpitation), cardiovascular risk factors (hypertension, diabetes, tobacco use, alcohol misuse, dyslipidemia, sedentarity), history of heart failure and other vascular events, anthropometric parameters (weight, height, waist circumference), hemodynamic parameters (blood pressure, pulses, respiratory rate), and clinical signs of left and right heart failure.
Measurements
Echocardiography was performed with the patient in the left lateral decubitus position using two commercially available echocardiographs (Philips and Hitachi). The type or mechanism of MR was classified according to Carpentier classification (9). We quantified secondary MR using the size of the regurgitant orifice (SOR) expressed in millimeter square (mm2). SOR was obtained using the formula: SOR = 2 × π × r2 × V/Vmax, where r is the radius of the Proximal Isovelocity Surface Area (PISA), V is the aliasing velocity, Vmax is the maximal velocity of MR. The regurgitant volume expressed as mL/beat was derived by multiplying SOR with the velocity time integral of MR obtained with continuous Doppler.
Left ventricular end-diastolic diameter (LVEDd) expressed in millimeters was measured in the long parasternal window view. Patients with LVEDd ≥60 mm were retained for the study. Left atrial surface area was measured in end-systole in the apical four chamber view. Left atrial enlargement was considered if this was ≥20 cm2. Right ventricular diameter (mm) was measured in the long parasternal window view. This was considered dilated when it was >45 mm. Left ventricular ejection fraction (EF) was measured using the Teicholz method. This was considered normal if EF ≥50%. Diastolic function was assessed from the trans-mitral pulse wave and lateral mitral annulus tissue Doppler measurements. This was classified using the Appleton classification (10).
Working definitions
Hypertensive heart disease (HHD) was diagnosed in patients with hypertension and eccentric left ventricular hypertrophy on echocardiography. Ischemic heart disease (IHD) was diagnosed when there was a history suggestive of IHD, ECG signs of ischemia or tissue necrosis, and regional wall motion anomaly on echocardiography. Peripartum cardiomyopathy was diagnosed as dilated heart disease in the peripartum period. Specific metabolic causes include the presence of diabetes and amyloidosis. Primary dilated cardiomyopathy was diagnosed when there was no identifiable cause.
Study size
Patients were consecutively recruited for the study during the period of research. A consecutive sample of all possible eligible patients was considered.
Statistical methods
Data was analyzed with the Statistical Package for Social Sciences software (SPSS) version 20.0 (IBM Corp. Released 2012). Categorical variables were expressed as frequencies and proportions, and continuous variables as means (SD) and scatter plots. We grouped patients in to two, those with severe MR and those without severe MR and compared their echocardiographic characteristics using Student t-test. We studied the correlations of the severity of MR with age, sex, blood pressure, and some echocardiographic parameters such as chamber size, ventricular functions, and pulmonary pressures using Pearson correlation coefficient. A P value < 0.05 was considered statistically significant for observed difference or trends.
Results
Participants and descriptive data
A total of 2,240 echocardiographies were performed, of whom 25 (1.12%) participants (13 males, 52%) with an echocardiographic diagnosis of dilated left ventricle were included in the study. Their mean age was 53.2 years (range: 22 to 84 years). The most represented age group were those 50 to 60 years for those with non-severe MR and 60 to 70 years for those with severe MR. Those with severe MR were significantly older (mean age: 63.8±10.8 versus 47.7±11.26 years, P=0.013). The clinical characteristics of the study population are shown in Table 1.
Table 1
| Clinical parameter | Frequency (n) | Percentage (%) |
|---|---|---|
| Cardiovascular risk factors | ||
| Hypertension | 13 | 52 |
| Diabetes | 11 | 44 |
| Alcohol consumption | 15 | 60 |
| Tobacco use | 7 | 28 |
| Obesity | 3 | 12 |
| Sedentarity | 8 | 32 |
| Dyslipidemia | 6 | 24 |
| Medical history | ||
| Coronary syndrome | 4 | 16 |
| Heart failure | 10 | 40 |
| Symptoms | ||
| Dyspnea grade II–IV | 23 | 92 |
| Orthopnea | 18 | 72 |
| Palpitation | 13 | 52 |
| Chest pain | 3 | 12 |
| Cough | 8 | 32 |
| Hepatalgia | 1 | 4 |
| Asthenia | 16 | 64 |
| Signs of right heart failure | ||
| Distended neck veins | 8 | 32 |
| Abdominal-jugular reflux | 7 | 28 |
| Pedal edema | 16 | 64 |
| Congestive hepatomegaly | 5 | 20 |
| Ascites | 3 | 12 |
| Signs of left heart failure | ||
| Tachycardia | 9 | 36 |
| S3 Gallop | 2 | 8 |
| S4 Gallop | 1 | 4 |
| Loud pulmonic S2 | 1 | 4 |
| Mitral regurgitation murmur | 11 | 44 |
| Pulmonary crepitation | 7 | 28 |
| Pleural effusion | 1 | 4 |
The most frequent risk factors for heart disease were alcohol use and hypertension. Forty percent (40%) of the patients had a history of heart failure. Dyspnea (grade II–IV), orthopnea, and asthenia were the most frequent symptoms. Bilateral pedal edema and murmur of mitral regurgitation (MR) were the most frequent signs. Of those with MR murmur [11 (44%)], 6 had grade 3/6 and 5 had grade 4/6 murmur.
Outcome data and main results
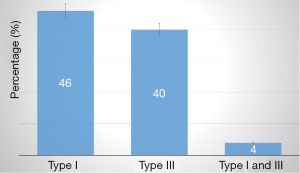
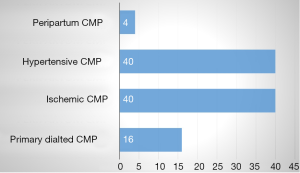
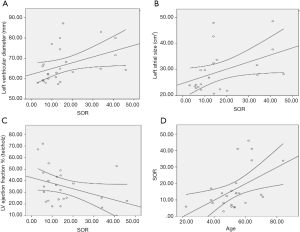
All patients with dilated LV had MR, of whom 6 (24%) had severe MR (grade IV). 19 patients had MR grade I to II. The mechanism of MR according to Carpentier is shown in Figure 1. Type I MR was seen in 14 (46%), type III in 10 (40%), and type I and III in 1 (4%) of patients. The aetiologies of secondary MR are shown in Figure 2. The baseline heart disease were primary 4 (16%), ischemic 10 (40%), hypertensive 10 (40%), and peripartum cardiomyopathy. The echocardiographic parameters are shown in Table 2. The left atrium was dilated in 22 (88%), raised filling pressure in 19 (76%), and raised pulmonary pressure in 15 (60%). All patients had LV systolic dysfunction. Compared with those who had mild to moderate MR, those with severe MR had lower LV ejection fraction and right atrial size. They also had higher LV end diastolic diameter, larger left atrial size, and higher pulmonary pressures (all P values >0.05). The electrocardiographic parameters (n=21) are shown in Table 3. The most frequent anomaly was Left Ventricular hypertrophy and T-wave inversion. The correlates of secondary MR are shown in Figure 3. The severity of MR significantly correlated with age (r=0.5, P=0.011), left ventricle end diastolic diameter (r=0.41, P=0.04), low ejection fraction (r=−0.378, P=0.031), and left atrial size (r=0.431, P=0.022). The severity of MR did not correlate with systemic blood pressure (P=0.800), right atrial size (P=0.419), and pulmonary systolic pressure (P=0.349). Cardiomegaly was constantly seen on chest X-ray (n=19). The cardiothoracic index was similar between those with moderate and those with severe MR. Pleural effusion on chest X-ray was seen in 6 (31.5%) of patients, all of whom had moderate MR.
Table 2
| Parameter | Mean (standard deviation) | P value | ||
|---|---|---|---|---|
| Overall | Severe MR | Mild/moderate MR | ||
| Left ventricular end-diastolic diameter (mm) | 66.7 (8.6) | 71.1 (8.1) | 65.4 (8.4) | 0.160 |
| Left atrial surface (cm2) | 29.5 (8.3) | 32.7 (9.3) | 28.2 (7.7) | 0.260 |
| Right atrial surface (cm2) | 24.9 (2.6) | 24.2 (2.1) | 25.4 (2.7) | 0.460 |
| Regurgitant surface (mm2)) | 16.4 (12.5) | 36 (9.0) | 10.1 (4.1) | <0.001 |
| Regurgitant volume (cc per heart beat) | 21.8 (20.6) | 37.4 (12.3) | 12.2 (6.5) | <0.001 |
| Left ventricular ejection fraction (%) | 34.9 (15.9) | 27.6 (15.3) | 37.2 (15.7) | 0.205 |
| Left ventricular fractional shortening (%) | 20.1 (11.7) | 14.8 (7.8) | 21.8 (12.3) | 0.209 |
| Pulmonary systolic pressure (mmHg) | 61.9 (10.9) | 65.1 (5.6) | 59.7 (12.2) | 0.377 |
Table 3
| Parameter | Frequency (n) | Percentage (%) |
|---|---|---|
| Normal sinus rhythm | 20 | 95.2 |
| Sinus tachycardia | 4 | 19 |
| Atrial fibrillation | 1 | 4.7 |
| Premature ventricular beat | 8 | 38.1 |
| Normal A-V conduction | 14 | 66.6 |
| Complete left bundle branch block | 7 | 33.3 |
| Left ventricular hypertrophy | 14 | 66.6 |
| Left atrial enlargement | 6 | 28.5 |
| Right atrial enlargement | 1 | 4.7 |
| Pathologic Q waves | 5 | 23.8 |
| ST segment elevation | 2 | 9.5 |
| T wave inversion | 13 | 61.9 |
Discussion
Key results
Dilated cardiopathy is frequently associated with secondary MR (9). MR has been shown to have prognostic implications that can be modified with appropriate and timely treatment (1). We carried out this cross-sectional hospital based study to assess the occurrence and correlates of secondary MR in adult patients with dilated left ventricles of varying aetiologies in sub-Saharan Africa (SSA). This occurred in about 1.12% of echocardiograms. Secondary MR is almost always present in patients with dilated left ventricles. The most frequent mechanisms of secondary MR are type I and type III according to Carpentier. The severity of MR significantly correlated with age, low ejection fraction, left ventricular end diastolic diameter, and left atrial size. Several morbi-mortality and therapeutic outcome studies of secondary MR have been reported in the literature, but mostly in patients with IHD. Little is known on the occurrence, mechanism and correlates of secondary MR in SSA.
Limitations
This work should be interpreted in the light of some limitations, the small sample size due to the very selective population and the fact that this was not a community based study (11). Thus, we could not present the burden of MR at the community level. With the highly selective nature of the study population, we could not give a clear view of secondary MR in those with possible secondary MR without LV dilation like in hypertrophic and obstructive heart disease. However, this prospective echocardiography study aimed at providing baseline data on the etiologies of secondary MR and its correlates in indigenous SSA.
Interpretation
Compared to data from high income settings (11), our series were relatively younger, and both sexes were equally affected. This implied a higher burden of the disease condition affecting the working age group that is also pressed with the communicable diseases. Types I and Type III MR occurred almost equally in our series. We expected more cases of Type I MR in our setting due to the high prevalence rates of HHD and primary dilated cardiomyopathies (12-14).
HHD and primary dilated cardiomyopathy will result in global left ventricular remodeling and the dilation of the mitral annulus, and MR will be due to co-aptation defect or tenting of the mitral valve leaflets (Type I MR) (15). In high-income settings, more cases of Type III MR are recorded due to the higher rates of IHD (9). This will result in regional left ventricular remodeling and dysfunction of the sub-valvular apparatus, and MR will be due to malfunction of a valve leaflet (Type III MR).
This suggests that, IHD that used to be rare in our setting is becoming more frequent, thus a double burden of heart disease in low-income settings. Also, cases of IHD can be misdiagnosed as gastro-duodenal ulcer disease (16), and may die before the diagnosis is ever made. Secondary MR appeared to be a marker of disease severity as it significantly correlated with LV systolic dysfunction and dilation the left heart cavities in our series. Those with severe MR had lower LV ejection fraction, more dilated LV, larger left atrial size, and higher pulmonary pressures. These have all been shown to be associated with worse short and long term outcomes (9,17). Thus the severity of MR is a surrogate maker of cardiovascular disease outcome, and timely and appropriate treatment of MR has been shown to improve outcome (1,3,9). This stresses the need to actively screen for MR using advanced technologies such as cardiac ultrasound, and institute treatment accordingly. The diagnosis of MR using the auscultation method appeared to be insufficient (11). Only 44% of patients in our series had murmur of MR, all of medium-to-high grades. Also, this method cannot determine the severity of MR, necessary for the appropriate treatment.
Conclusions
Secondary MR is almost always present in patients with dilated cardiopathy. Hypertensive and IHD were the most frequent causes of dilated cardiopathy. Type I MR according to Carpentier classification was the most frequent mechanism of MR in our setting. The severity of MR correlated with age, low ejection fraction, left ventricular end diastolic diameter, and left atrial size. In other to reduce morbidity and mortality in low income settings, emphasis should be placed on preventive measures to reduce hypertension, a major cause of HHD and secondary MR. Also, with aging of the population and the rising trend in IHD, there is the need to screen for secondary MR and put in place the required know how to treat this condition.
Acknowledgments
We thank the support staff of the echocardiography laboratory of the Yaounde Central and General Hospitals.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2017.03.13). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical clearance was obtained from the ethical committee of the Faculty of Medicine and Biomedical Sciences, University of Yaounde 1 and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wu AH, Aaronson KD, Bolling SF, et al. Impact of mitral valve annuloplasty on mortality risk in patients with mitral regurgitation and left ventricular systolic dysfunction. J Am Coll Cardiol 2005;45:381-7. [Crossref] [PubMed]
- Digiammarco G, Liberi R, Giancane M, et al. Recurrence of functional mitral regurgitation in patients with dilated cardiomyopathy undergoing mitral valve repair: how to predict it. Interact Cardiovasc Thorac Surg 2007;6:340-4. [Crossref] [PubMed]
- Nicolini F, Agostinelli A, Vezzani A, et al. Surgical treatment for functional ischemic mitral regurgitation: current options and future trends. Acta Biomed 2015;86:17-26. [PubMed]
- Cioffi G, Tarantini L, De Feo S, et al. Functional mitral regurgitation predicts 1-year mortality in elderly patients with systolic chronic heart failure. Eur J Heart Fail 2005;7:1112-7. [Crossref] [PubMed]
- Timek TA, Miller DC. Another multidisciplinary look at ischemic mitral regurgitation. Semin Thorac Cardiovasc Surg 2011;23:220-31. [Crossref] [PubMed]
- Feldman T, Kar S, Rinaldi M, et al. Percutaneous mitral repair with the MitraClip system: safety and midterm durability in the initial EVEREST (Endovascular Valve Edge-to-Edge REpair Study) cohort. J Am Coll Cardiol 2009;54:686-94. [Crossref] [PubMed]
- World Medical Association. Declaration of Helsinki. Law Med Health Care 1991;19:264-5. [PubMed]
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Bull World Health Organ 2007;85:867-72. [Crossref] [PubMed]
- Bursi F, Enriquez-Sarano M, Jacobsen SJ, et al. Mitral regurgitation after myocardial infarction: a review. Am J Med 2006;119:103-12. [Crossref] [PubMed]
- Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 2009;10:165-93. [Crossref] [PubMed]
- Bursi F, Enriquez-Sarano M, Nkomo VT, et al. Heart failure and death after myocardial infarction in the community: the emerging role of mitral regurgitation. Circulation 2005;111:295-301. [Crossref] [PubMed]
- Jingi AM, Noubiap JJ, Kamdem P, et al. The spectrum of cardiac disease in the West Region of Cameroon: a hospital-based cross-sectional study. Int Arch Med 2013;6:44. [Crossref] [PubMed]
- Touze JE, Fourcade L. Cardiomyopathies in tropical countries: Causes and nosological perspective. World J Cardiovasc Surg 2013;3:201-8. [Crossref]
- Tantchou Tchoumi JC, Ambassa JC, Kingue S, et al. Occurrence, aetiology and challenges in the management of congestive heart failure in sub-Saharan Africa: experience of the Cardiac Centre in Shisong, Cameroon. Pan Afr Med J 2011;8:11. [PubMed]
- Yiu SF, Enriquez-Sarano M, Tribouilloy C, et al. Determinants of the degree of functional mitral regurgitation in patients with systolic left ventricular dysfunction: A quantitative clinical study. Circulation 2000;102:1400-6. [Crossref] [PubMed]
- Nkoke C, Luchuo EB. Coronary heart disease in sub-Saharan Africa: still rare, misdiagnosed or underdiagnosed? Cardiovasc Diagn Ther 2016;6:64-6. [PubMed]
- Bursi F, McNallan SM, Redfield MM, et al. Pulmonary pressures and death in heart failure: a community study. J Am Coll Cardiol 2012;59:222-31. [Crossref] [PubMed]
Cite this article as: Hamadou B, Boombhi J, Yowo LA, Ndongo Amougou S, Menanga A, Kingue S. Aetiologies and correlates of secondary mitral regurgitation in patients with dilated cardiopathy in Cameroon: a cross-sectional study. J Xiangya Med 2017;2:37.