Global microRNA downregulation: all roads lead to estrogen
Introduction
MicroRNAs (miRNAs) are endogenous small noncoding segments of RNA, ~22 nucleotides (nt) in length, that negatively regulate gene expression at the post-transcriptional level and have a role in fine-tuning gene expression in the cells (1). After being transcribed by RNA polymerase II, the primary miRNA transcripts (pri-miRNAs) are first processed by the RNase-III endonuclease Drosha and its associated binding partner DGCR8, which cut the pri-miRNAs into ~70-nt stem and loop miRNA precursors (pre-miRNAs), containing the mature miRNA sequence in one of its arms and the less abundant partially complementary miRNA mature form in the other arm (2,3). After the first processing step, pre-miRNAs are actively transported from the nucleus to the cytoplasm by the RanGTP-dependent RNA-binding protein (RBP) Exportin-5, where they are processed by another RNase-III endonuclease termed Dicer (4). The result of this processing event is a double stranded RNA, where one of its strands is incorporated into the argonaute (Ago) protein of the RNA-induced silencing complex (RISC), that based on the miRNA sequence, targets it to a 3’ untranslated region (3’UTR) of a specific mRNA and leads to its degradation (5). MiRNA target recognition mostly depends on the seed region (nt 2–8 from the 5' end of miRNA) (1). According to computational miRNA target prediction programs each miRNA can potentially regulate the expression of hundreds of different genes and it is therefore becoming increasingly apparent that miRNAs are involved in almost every cellular process investigated so far, and in the development of many human diseases.
miRNA repression associated with carcinogenesis
A massive downregulation of miRNAs was observed in human cancers, where miRNAs show lower expression levels in tumors compared with normal tissues (6). In their study, Lu et al. (6) determined the expression pattern of miRNAs across a large panel of samples representing diverse human tissues and tumor types, including colon, kidney, prostate, bladder, uterus, lung and breast, and observed differential expression of many miRNAs across the aforementioned cancer types. Most striking was their observation that most of the differentially expressed miRNAs had lower expression levels in tumors compared with normal tissues. Moreover, the same phenomenon was even more pronounced in aggressive poorly differentiated tumors (PDTs) of lung, breast and ovary, and was also seen in a mouse model of lung adenocarcinoma, which showed low miRNA expression relative to normal lungs (6).
The observation of a global miRNA repression in the lungs of rodents exposed to cigarette smoke (CS) has also been reported (7-10). Izzotti et al. show in their results the extensive down-regulation of 126 miRNAs in lungs of rats, 4 weeks after CS exposure (7). Such a short-lasting exposure to CS resulted in reversible miRNA alterations, as miRNA down-regulation was considerably attenuated one week after smoking cessation (9). By contrast, the repression of miRNA detected in mice exposed to CS for 4 months still persisted 3 months after smoking cessation, with the progressive development of cancer in the lung, suggesting that long-lasting exposure is needed to induce irreversible miRNA alterations (9,11). In previous published results, using the same animal model, Izzotti et al. have found that CS up-regulates gene transcription and protein expression (12,13). Similar results have also been published by Schembri et al. (14), who found that most of the differentially expressed miRNAs in the human bronchial airway epithelium were down-regulated in smokers and were inversely correlated with their predicted targets. The same phenomenon was also observed in alveolar macrophages of smokers, where CS decreased global miRNA expression, while increased their predicted targets (15). In this later study the decrease in global miRNA expression was more pronounced in heavy smokers, suggesting that the magnitude of miRNA repression is related to the extent of smoking history (15).
Izzotti et al. (16) evaluated miRNA expression in the lungs of rats exposed to CS and treated with several cancer chemopreventive agents. Administration of the dietary agents Phenethyl Isothiocyanate (PEITC) and Indole-3-Carbinol (I3C), two major components of cruciferous vegetables, attenuated the CS-induced down-regulation of miRNA expression. In the case of the combined treatment with PEITC and I3C, they had profound effects on almost all CS- down-regulated miRNAs and their expression even exceeded the baseline situation (16). Interestingly, both PEITC and I3C have proved to be anti-estrogenic compounds and inhibited ER-alpha expression (17-20). A similar effect was seen when mice were treated with the anti-diabetic drug Metformin (21). Exposure of mice to CS resulted in down-regulation of miRNA expression in the lungs. Metformin effectively changed the miRNA alterations resulted from exposure to CS in the mouse lung and normalized the expression of several down-regulated miRNAs (21). Intriguingly, there is evidence that Metformin is also an anti-estrogenic molecule; it was shown to inhibit ER-alpha expression in cancer cells and to decrease estrogen levels in the serum of breast cancer female patients (22,23).
These results raise the possibility that the hormone estrogen might regulate the CS-induced miRNA alterations in the lungs (24). Estrogens and their receptors were detected within murine lung tissue (25,26), and there is evidence for higher susceptibility of women to smoking-related lung cancer (27-29), and that anti-estrogens can prevent lung tumorigenesis (30). Moreover, CS was found to accelerate the production of the carcinogenic estrogenic metabolites 4-OHEs in the lungs (26), and the estrogen-metabolizing enzyme Cytochrome P450 1b1 (CYP1B1) was shown to be consistently induced following exposure to CS (25,26,31,32).
microRNA repression associated with estrogen exposure
Several studies have demonstrated that exposure to the hormone estrogen leads to widespread repression in miRNA expression (33-36). Maillot et al. showed that the expression of 23 miRNAs decreases following 17beta-estradiol (E2) treatment and also showed the involvement of several of the repressed miRNAs in E2-dependent cell growth and proliferation (33). This is further supported by the study of Yamagata et al. who showed a similar repression in miRNA expression in E2-injected, ovariectomized mice (34).
We have shown the global miRNA downregulation in the zebrafish liver after E2 treatment (35). Since regulation by miRNAs resulted in miRNA-mediated gene repression, it can be assumed that the regulation by repressed miRNAs is weakened and as a consequence the mRNA stability of target genes is increased. Indeed, using the combination of computational prediction with data obtained from miRNAs and mRNAs microarrays, performed from the same biological samples, our results showed that putative targets of miRNAs predominantly tend to be upregulated after E2 treatment (35).
Estrogen can cause cellular growth, proliferation and cancer by inducing proto-oncogenes such as c-myc (37). C-Myc physically interacts with ER-alpha and is recruited to estrogen-responsive genes (38). Most interestingly, a widespread miRNA repression by c-Myc was also reported, and the observed substantial down-regulation of miRNAs was correlated with enhanced cellular proliferation and contributed to Myc-mediated tumorigenesis (39).
Mounting evidence suggests that oxidative metabolites may contribute to estrogen carcinogenesis (40). The potential carcinogenic activity of estrogen involves the oxidative metabolism of estrone (E1) or E2 to catechol estrogens and the reactive quinone metabolites (41,42), that bind covalently with purines in DNA to form specific DNA adducts at the N-3 of adenine (Ade) and N-7 of guanine (Gua) (43,44). These adducts generate apurinic sites that can be converted into mutations by error-prone repair, which in turn may initiate tumorigenesis (45). Two types of DNA-adduct can be formed: a stable adduct and a depurinating adduct, where evidence indicates that the second one plays a major role in tumor initiation (46).
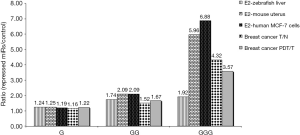
Izzotti and Pulliero (47) evaluated the formation of Gua-adducts in miRNAs of the lungs of mice that were exposed to CS and found that the Gua-adducts level was 5.7-fold higher than that detectable in the DNA of the same animals, and therefore concluded that miRNAs are more sensitive than DNA to the formation of adducts induced by exposure to CS (47). They also showed, using bioinformatic analysis, that the Gua content of the terminal loop (TL) of miRNAs that are involved in stress response is higher than the Gua content of the other miRNAs (47). We have revealed, using miRNA expression data analysis of zebrafish, the mouse and human MCF-7 cells, the association of estrogen-related miRNAs repression with a high Gua content in the TL sequences of their precursors (Figure 1) (48). Moreover, we also found a similar association, between the widespread miRNAs reduction that is observed in several types of human cancers and a high TL Gua content in their precursors (Figure 1) (49). These results suggest a possible link between miRNA-Gua adducts formation and carcinogenesis, while both CS and estrogen exposure form miRNA adducts that cause repression of tumor suppressor miRNAs and induction of their target oncogenes, which may ultimately lead to carcinogenesis (Figure 2). Indeed, several of the E2- and cancer-repressed miRNAs were also shown to function as tumor suppressors. For example, miR-15a, which was predominantly repressed in PDTs, is a well-known tumor suppressor (50,51), miRNAs of the let-7 family repress the expression of known oncogenes, including k-Ras and c-Myc (52,53), miR-143 and miR-145 are co-expressed miRNAs that function as tumor suppressors (54,55), and miR-26a was shown to strongly inhibit estrogen-stimulated breast cancer cells and tumor growth (33,56).
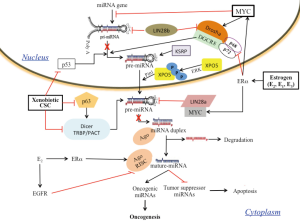
Findings suggest that the miRNA terminal loop is an important platform for different RBPs that act as activators or repressors of Drosha and Dicer processing, and selectivity regulate miRNAs by binding to the RNA TLs of their precursors (57). For example, it was shown that miRNAs with the tetra-nucleotide sequence motif GGAG in their TL were regulated through binding of the RBP Lin28, which interferes with Dicer processing (58). Our results revealed a high enrichment for the sequence motif GGAG in TLs of the E2- and cancer-repressed miRNAs (48,49). Also, a significant enrichment of triple Gua (GGG) motif in TLs of the E2- and cancer-repressed miRNAs (Figure 1) was observed. In their study, García-Mayoral et al. described the complete analysis of the RNA-binding potential of the four KH domains of KSRP and show that the KH3 domain is able to recognize a G-rich sequence (59). Insertion of an isolated G led to a 5-fold increase in KH3 affinity, whereas insertion of a GG element led to a further 4-fold increase (59). Most interestingly, KH3 binding docks KSRP to the GGG-containing TL of a subset of miRNAs and promotes their maturation (60). Therefore, Gua-adducts formation that disrupts binding of KSRP to the TL might be a possible cause for the observed E2- and cancer-repressed miRNAs. Of note, single nucleotide polymorphisms (SNPs) which involve A>G and G<A nucleotide transitions, and are located at the TL sequences of pre-miRNAs, were shown to be associated with the development of breast and gastric cancers (61,62).
Perspectives
The results showing global miRNA reduction after estrogen and CS exposure, and in different types of cancer, in association with Gua enrichment in the TLs of their precursors, suggest the involvement of estrogen-related pathways in these phenomena. Elucidating the molecular mechanisms of estrogens involved in miRNA repression, and revealing the target genes of the downregulated miRNAs, can help better understand the processes driving miRNA downregulation, and has potential implications for cancer therapy and prevention.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2017.07.03). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009;136:215-33. [Crossref] [PubMed]
- Zeng Y, Cullen BR. Recognition and cleavage of primary microRNA precursors by the nuclear processing enzyme Drosha. EMBO J 2005;24:138-48. [Crossref] [PubMed]
- Yang JS, Phillips MD, Betel D, et al. Widespread regulatory activity of vertebrate microRNA* species. RNA 2011;17:312-26. [Crossref] [PubMed]
- Cullen BR. Transcription and processing of human microRNA precursors. Mol Cell 2004;16:861-5. [Crossref] [PubMed]
- Jiang S, Yan W. Current view of microRNA processing. Sign Transduct Insights 2016;5:9-13. [Crossref]
- Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature 2005;435:834-8. [Crossref] [PubMed]
- Izzotti A, Calin GA, Arrigo P, et al. Downregulation of microRNA expression in the lungs of rats exposed to cigarette smoke. FASEB J 2009;23:806-12. [Crossref] [PubMed]
- Izzotti A, Calin GA, Steele VE, et al. Relationships of microRNA expression in mouse lung with age and exposure to cigarette smoke and light. FASEB J 2009;23:3243-50. [Crossref] [PubMed]
- Izzotti A, Larghero P, Longobardi M, et al. Dose-responsiveness and persistence of microRNA expression alterations induced by cigarette smoke in mouse lung. Mutat Res 2011;717:9-16. [Crossref] [PubMed]
- Izzotti A, Larghero P, Balansky R, et al. Interplay between histopathological alterations, cigarette smoke and chemopreventive agents in defining microRNA profiles in mouse lung. Mutat Res 2011;717:17-24. [Crossref] [PubMed]
- Izzotti A, Pulliero A. Molecular damage and lung tumors in cigarette smoke–exposed mice. Ann N Y Acad Sci 2015;1340:75-83. [Crossref] [PubMed]
- Izzotti A, Bagnasco M, Cartiglia C, et al. Chemoprevention of genome, transcriptome, and proteome alterations induced by cigarette smoke in rat lung. Eur J Cancer 2005;41:1864-74. [Crossref] [PubMed]
- Izzotti A, Bagnasco M, Cartiglia C, et al. Modulation of multigene expression and proteome profiles by chemopreventive agents. Mutat Res 2005;591:212-23. [Crossref] [PubMed]
- Schembri F, Sridhar S, Perdomo C, et al. MicroRNAs as modulators of smoking-induced gene expression changes in human airway epithelium. Proc Natl Acad Sci U S A 2009;106:2319-24. [Crossref] [PubMed]
- Graff JW, Powers LS, Dickson AM, et al. Cigarette smoking decreases global microRNA expression in human alveolar macrophages. PLoS One 2012;7:e44066. [Crossref] [PubMed]
- Izzotti A, Calin GA, Steele VE, et al. Chemoprevention of cigarette smoke-induced alterations of MicroRNA expression in rat lungs. Cancer Prev Res (Phila) 2010;3:62-72. [Crossref] [PubMed]
- Kang L, Ding L, Wang ZY. Isothiocyanates repress estrogen receptor alpha expression in breast cancer cells. Oncol Rep 2009;21:185-92. [PubMed]
- Kang L, Wang ZY. Breast cancer cell growth inhibition by phenethyl isothiocyanate is associated with down-regulation of oestrogen receptor-alpha36. J Cell Mol Med 2010;14:1485-93. [Crossref] [PubMed]
- Sundar SN, Kerekatte V, Equinozio CN, et al. Indole-3-carbinol selectively uncouples expression and activity of estrogen receptor subtypes in human breast cancer cells. Mol Endocrinol 2006;20:3070-82. [Crossref] [PubMed]
- Meng Q, Yuan F, Goldberg ID, et al. Indole-3-carbinol is a negative regulator of estrogen receptor-alpha signaling in human tumor cells. J Nutr 2000;130:2927-31. [PubMed]
- Izzotti A, Balansky R, D'Agostini F, et al. Modulation by metformin of molecular and histopathological alterations in the lung of cigarette smoke-exposed mice. Cancer Med 2014;3:719-30. [Crossref] [PubMed]
- Kim J, Lee J, Jang S, et al. The anti-estrogen effect of metformin in ERα+ breast cancer and tamoxifen resistant cell lines. Cancer Res 2014;74:Abstract nr 4229.
- Campagnoli C, Berrino F, Venturelli E, et al. Metformin decreases circulating androgen and estrogen levels in non-diabetic women with breast cancer. Clin Breast Cancer 2013;13:433-8. [Crossref] [PubMed]
- Cohen A, Burgos-Aceves MA, Smith Y. A potential role for estrogen in cigarette smoke-induced microRNA alterations and lung cancer. Transl Lung Cancer Res 2016;5:322-30. [Crossref] [PubMed]
- Meireles SI, Esteves GH, Hirata R Jr, et al. Early changes in gene expression induced by tobacco smoke: Evidence for the importance of estrogen within lung tissue. Cancer Prev Res (Phila) 2010;3:707-17. [Crossref] [PubMed]
- Peng J, Xu X, Mace BE, et al. Estrogen metabolism within the lung and its modulation by tobacco smoke. Carcinogenesis 2013;34:909-15. [Crossref] [PubMed]
- Siegfried JM. Women and lung cancer: does oestrogen play a role? Lancet Oncol 2001;2:506-13. [Crossref] [PubMed]
- Gasperino J, Rom WN. Gender and lung cancer. Clin Lung Cancer 2004;5:353-9. [Crossref] [PubMed]
- Gasperino J. Gender is a risk factor for lung cancer. Med Hypotheses 2011;76:328-31. [Crossref] [PubMed]
- Burns TF, Stabile LP. Targeting the estrogen pathway for the treatment and prevention of lung cancer. Lung Cancer Manag 2014;3:43-52. [Crossref] [PubMed]
- Han W, Pentecost BT, Pietropaolo RL, et al. Estrogen receptor alpha increases basal and cigarette smoke extract-induced expression of CYP1A1 and CYP1B1, but not GSTP1, in normal human bronchial epithelial cells. Mol Carcinog 2005;44:202-11. [Crossref] [PubMed]
- Siegfried JM. Early changes in pulmonary gene expression following tobacco exposure shed light on the role of estrogen metabolism in lung carcinogenesis. Cancer Prev Res (Phila) 2010;3:692-5. [Crossref] [PubMed]
- Maillot G, Lacroix-Triki M, Pierredon S, et al. Widespread estrogen-dependent repression of micrornas involved in breast tumor cell growth. Cancer Res 2009;69:8332-40. [Crossref] [PubMed]
- Yamagata K, Fujiyama S, Ito S, et al. Maturation of microRNA is hormonally regulated by a nuclear receptor. Mol Cell 2009;36:340-7. [Crossref] [PubMed]
- Cohen A, Smith Y. Estrogen regulation of microRNAs, target genes, and microRNA expression associated with vitellogenesis in the zebrafish. Zebrafish 2014;11:462-78. [Crossref] [PubMed]
- Cohen A, Burgos-Aceves MA, Smith Y. Estrogen repression of microRNA as a potential cause of cancer. Biomed Pharmacother 2016;78:234-8. [Crossref] [PubMed]
- Musgrove EA, Sergio CM, Loi S, et al. Identification of functional networks of estrogen- and c-Myc-responsive genes and their relationship to response to tamoxifen therapy in breast cancer. PLoS ONE 2008;3:e2987. [Crossref] [PubMed]
- Cheng AS, Jin VX, Fan M, et al. Combinatorial analysis of transcription factor partners reveals recruitment of c-MYC to estrogen receptor-alpha responsive promoters. Mol Cell 2006;21:393-404. [Crossref] [PubMed]
- Chang TC, Yu D, Lee YS, et al. Widespread microRNA repression by Myc contributes to tumorigenesis. Nat Genet 2008;40:43-50. [Crossref] [PubMed]
- Yager JD. Endogenous estrogens as carcinogens through metabolic activation. J Natl Cancer Inst Monogr 2000;27:67-73. [Crossref] [PubMed]
- Gaikwad NW. Metabolomic profiling unravels DNA adducts in human breast that are formed from peroxidase mediated activation of estrogens to quinone methides. PLoS ONE 2013;8:e65826. [Crossref] [PubMed]
- Yang L, Zahid M, Liao Y, et al. Reduced formation of depurinating estrogen–DNA adducts by sulforaphane or KEAP1 disruption in human mammary epithelial MCF-10A cells. Carcinogenesis 2013;34:2587-92. [Crossref] [PubMed]
- Cavalieri E, Rogan E. Catechol quinones of estrogens in the initiation of breast, prostate, and other human cancers: keynote lecture. Ann N Y Acad Sci 2006;1089:286-301. [Crossref] [PubMed]
- Belous AR, Hachey DL, Dawling S, et al. Cytochrome P450 1B1-mediated estrogen metabolism results in estrogen-deoxyribonucleoside adduct formation. Cancer Res 2007;67:812-7. [Crossref] [PubMed]
- Cavalieri E, Chakravarti D, Guttenplan J, et al. Catechol estrogen quinones as initiators of breast and other human cancers: implications for biomarkers of susceptibility and cancer prevention. Biochim Biophys Acta 2006;1766:63-78.
- Cavalieri EL, Rogan EG. Depurinating estrogen-DNA adducts, generators of cancer initiation: their minimization leads to cancer prevention. Clin Transl Med 2016;5:12. [Crossref] [PubMed]
- Izzotti A, Pulliero A. The effects of environmental chemical carcinogens on the microRNA machinery. Int J Hyg Environ Health 2014;217:601-27. [Crossref] [PubMed]
- Cohen A, Burgos-Aceves MA, Kahan T, et al. Estrogen repression of microRNAs is associated with high guanine content in the terminal loop sequences of their precursors. 2017. Submitted for publication.
- Cohen A, Burgos-Aceves MA, Smith Y. microRNAs downregulation in cancer is associated with guanine enrichment in the terminal loop sequences of their precursors. 2017. Submitted for publication.
- Aqeilan RI, Calin GA, Croce CM. miR-15a and miR-16-1 in cancer: discovery, function and future perspectives. Cell Death Differ 2010;17:215-20. [Crossref] [PubMed]
- Huang E, Liu R, Chu Y. miRNA-15a/16: as tumor suppressors and more. Future Oncol 2015;11:2351-63. [Crossref] [PubMed]
- Wang X, Cao L, Wang Y, et al. Regulation of let-7 and its target oncogenes Oncol Lett 2012;3:955-60. (Review). [PubMed]
- He XY, Chen JX, Zhang Z, et al. The let-7a microRNA protects from growth of lung carcinoma by suppression of k-Ras and c-Myc in nude mice. J Cancer Res Clin Oncol 2010;136:1023-8. [Crossref] [PubMed]
- Kent OA, Chivukula RR, Mullendore M, et al. Repression of the miR-143/145 cluster by oncogenic Ras initiates a tumor-promoting feed-forward pathway. Genes Dev 2010;24:2754-9. [Crossref] [PubMed]
- Cui SY, Wang R, Chen LB. MicroRNA-145: a potent tumour suppressor that regulates multiple cellular pathways. J Cell Mol Med 2014;18:1913-26. [Crossref] [PubMed]
- Tan S, Ding K, Li R, et al. Identification of miR-26 as a key mediator of estrogen stimulated cell proliferation by targeting CHD1, GREB1 and KPNA2. Breast Cancer Res 2014;16:R40. [Crossref] [PubMed]
- Libri V, Miesen P, van Rij RP, et al. Regulation of microRNA biogenesis and turnover by animals and their viruses. Cell Mol Life Sci 2013;70:3525-44. [Crossref] [PubMed]
- Heo I, Joo C, Kim YK, et al. TUT4 in concert with Lin28 suppresses microRNA biogenesis through pre-microRNA uridylation. Cell 2009;138:696-708. [Crossref] [PubMed]
- García-Mayoral MF, Díaz-Moreno I, Hollingworth D, et al. The sequence selectivity of KSRP explains its flexibility in the recognition of the RNA targets. Nucleic Acids Res 2008;36:5290-6. [Crossref] [PubMed]
- Trabucchi M, Briata P, Garcia-Mayoral M, et al. The RNA-binding protein KSRP promotes the biogenesis of a subset of microRNAs. Nature 2009;459:1010-4. [Crossref] [PubMed]
- Yang R, Schlehe B, Hemminki K, et al. A genetic variant in the pre-miR-27a oncogene is associated with a reduced familial breast cancer risk. Breast Cancer Res Treat. 2010;121:693-702. [Crossref] [PubMed]
- Fernandez N, Cordiner RA, Young RS, et al. Genetic variation and RNA structure regulate microRNA biogenesis. Nat Commun 2017;8:15114. [Crossref] [PubMed]
Cite this article as: Cohen A, Burgos-Aceves MA, Smith Y. Global microRNA downregulation: all roads lead to estrogen. J Xiangya Med 2017;2:59.